Abstract
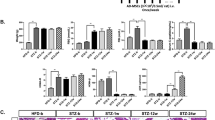
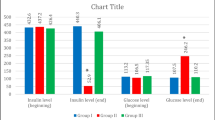
Diabetes mellitus (DM) is associated with severe progressive degenerative complications in many organs especially diabetic nephropathy (DN). Adipose-derived stromal vascular fraction (SVF) is easily obtained and has abundant viable stem cell number obviating extensive expansion in culture; thus, SVF utilization provides promising anticipation. Low-intensity laser irradiation (LILI) was found to strengthen the therapeutic effect of non-expanded SVF through enhancing viability, protein expression, and migration of stem cells. The current study aimed to demonstrate the effect of transplanted laser-activated SVF in streptozotocin (STZ)-induced diabetic rats. Forty-five male Sprague Dawley rats were used in this experiment and divided randomly into four groups: (I) control group, (II) diabetic untreated group, (III) and (IV) diabetic-treated groups in which laser-activated SVF transplantation performed as a single and multiple IP injections, respectively, (V) cell tracking group. DM was induced by a single intraperitoneal (IP) injection of STZ at a dose of 55 mg/kg. Rats received single and multiple IP SVF injections, at a dose of 1.5 × 106 nucleated cells/rat on the 7th day of DM induction and a second dose injected in group IV on the 21th day of DM induction (2-week interval). Insulin gene expression quantification; glycemic profile evaluation (blood glucose, C-peptide, and glycosylated hemoglobin); biochemical, histopathological, and immunohistochemical parameters; and microalbuminuria were assessed in all experimental groups. Both SVF-treated groups exhibited improvement in glycemic profile which was confirmed by the significant increase of insulin gene expression and C-peptide levels. Liver transaminases, lipid profile, and microalbuminuria were normalized while serum creatinine and urea concentrations were ameliorated in treated groups compared to diabetic untreated rats. In conclusion, laser-activated SVF transplantation in diabetic rats triggered improvement of the diabetic state and ameliorated some of its complications with regard to the early interference. The second SVF treatment showed more improvement regarding the blood glucose, C-peptide, histopathology, and immunohistochemical results of the pancreas in diabetic states.






Similar content being viewed by others
References
Abdallah AN, Shamaa AA, El-Tookhy OS, Abd El-Mottaleb EM (2016) Evaluation of low level laser-activated stromal vascular fraction as a single procedure for treatment of experimental chondral defects. Asian J Anim Sci 10:15–28
Abdel Aziz MT, Ei-Asmar MF, Haidara M, Atta HM, Roshdy NK, Rashed LA, Sabry D, Youssef MA, Abdel Aziz AT, Moustafa M (2008) Effect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic rats. Med Sci Monit 14:249–255
Abdel Aziz MT, Wassef MA, Ahmed HH, Rashed L, Mahfouz S, Aly MI, Hussein RE, Abdelaziz M (2014) The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr 6:1–10
Abdelrahim EA (2013) Histopathological change of the endocrine pancreas in male albino rat treated with the atypical antipsychotic clozapine. Romanian J Morphol Embryol 54:385–394
Abrahamse H (2011) Inducing stem cell differentiation using low intensity laser irradiation: a possible novel therapeutic intervention. Cent Eur J Biol 6:695–698
Al-Mahmood SM, Razak TA, Abdullah ST, Ahmad NN, Mohamed AH, Al-ani IM (2016) A comprehensive study of chronic diabetes complications in streptozotocin-induced diabetic rat. Makara J Heal Res 20:48–56
Bancroft J, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, Elsevier, China
Bell GI, Broughton HC, Levac KD, Allan DA, Xenocostas A, Hess DA (2012) Transplanted human bone marrow progenitor subtypes stimulate endogenous islet regeneration and revascularization. Stem Cells Dev 21:97–109
Bhansali S, Kumar V, Saikia UN, Medhi B, Jha V (2015) Effect of mesenchymal stem cells transplantation on glycaemic profile and their localization in streptozotocin induced diabetic Wistar rats. Indian J Med Res142:63–71
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347
Cardinali A, Nason G (2013) Costationarity of locally stationary time series using costat. J Stat Softw 55:1–22
Chen YF, Shibu MA, Fan MJ, Chen MC, Viswanadha VP, Lin YL et al (2016) Purple rice anthocyanin extract protects cardiac function in STZ-induced diabetes rat hearts by inhibiting cardiac hypertrophy and fibrosis. J Nutr Biochem 3:98–105
Davey GC, Patil SB, O’Loughlin A, O’Brien T (2014) Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus. Front Endocrinol 5:1–16
Dombrowski F, Klotz L, Bannasch P, Evert M (2007) Renal carcinogenesis in models of diabetes in rats - metabolic changes are closely related to neoplastic development. Diabetologia 50:2580–2590
Eissa CS, Abd-elhady Z, Fargali LM, Hosny S (2018) Effect of losartan on islets of langerhans β-cells streptozotocin-induced diabetes in adult male albino rats. Pharmacologia 1–12
El Barky AR, Ezz AA, Alm-Eldeen AA, Hussein SA, Hafez YA, Mohamed TM (2018) Can stem cells ameliorate the pancreatic damage induced by streptozotocin in rats? Can J Diabetes 42:61–70
El-domouky AM, Hegab AS, Al-shahat A, Raafat N (2017) Mesenchymal stem cells and differentiated insulin producing cells are new horizons for pancreatic regeneration in type I diabetes mellitus. Int J Biochem Cell Biol 87:77–85
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA (2008) Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14:631–640
Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H, Li D (2012) Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med 30:85–92
Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, Bube A (2012) Proliferative and nonproliferative lesions of the rat and mouse urinary system. Tox Path 40:14–86
Gabr MM, Sobh MM, Zakaria MM, Refaie AF, Ghoneim MA (2008) Transplantation of insulin-producing clusters derived from adult bone marrow stem cells to treat diabetes in rats. Exp Clin Transplant 6:236–243
Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Khater SM, Ashamallah SA, Azzam MM, Ghoneim MA (2013) Insulin-producing cells from adult human bone marrow mesenchymal stromal cells could control streptozotocin- induced diabetes in nude mice. Cell Transplant 22:133–145
Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO (2016) Challenges and issues with streptozotocin-induced diabetes: a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact 244:49–63
Hamza AH, Al-bishri WM, Damiati LA, Ahmed HH (2017) Mesenchymal stem cells :a future experimental exploration for recession of diabetic nephropathy. Ren Fail 3:67–76
Houlihan DD, Newsome PN (2008) Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroentrology 135:438–450
Hu J, Fu Z, Chen Y, Tang N, Wang L, Wang F, Sun R, Yan S (2015) Effects of autologous adipose-derived stem cell infusion on type 2 diabetic rats. Endocr J 62:339–352
Hu J, Wang Y, Gong H, Yu C, Guo C, Wang F, Yan S, Xu H (2016) Long term effect and safety of Wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med 12:1857–1866
Ishimura D, Yamamoto N, Tajima K, Ohno A, Yamamoto Y, Washimi O, Yamada H (2008) Differentiation of adipose-derived stromal vascular fraction culture cells into chondrocytes using the method of cell sorting with a mesenchymal stem cell marker. Tohoku J Exp Med 216:149–156
Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, Shao Y, Yang S, Han ZC (2011) Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med China 5:94–100
Kang H, Yang C, Ahn H, Kang E, Hong E, Jeung (2014) Effects of xenoestrogens on streptozotocin-induced diabetic mice. J Physiol Pharmacol 65:273–282
Li P, Zhang R, Sun H, Chen L, Liu F, Yao C, Du M, Jiang X (2013) PKH26 can transfer to host cells in vitro and vivo. Stem Cells Dev 22:340–344
Little MH, Kairath P (2016) Regenerative medicine in kidney disease. Kidney Int 90:289–299
Liu Z, Li J, Li P, Bai M, Guo Y, Han M, Zhang F, Ahmed R, Jin S (2016a) Stem cell transplantation for the treatment of liver diseases: a systematic review and meta-analysis. Turk J Gastroenterol 27:499–508
Liu G-Y, Liu J, Wang Y, Liu Y, Shao Y, Han Y, Qin Y, Xiao F, Feili P, Zhao L, Gu E, Chen S, Gao L, Wu C, Hu X, Duan H (2016b) Adipose-derived mesenchymal stem cells ameliorate lipid metabolic disturbance in mice. Stem Cells Transl Med 5:1162–1170
Mousa F, Abdel-aziz KK, Abdel Gawad H, Mahmoud SS, Elgamel MS (2016) Bone marrow-derived mesenchymal stem cells infusion ameliorates hyperglycemia, dyslipidemia, liver and kidney functions in diabetic rats. Int J Sci Res 5:1624–1631
Pourghasem M, Shafi H, Babazadeh Z (2015) Histological changes of kidney in diabetic nephropathy. Caspian J Int Med 6:120–127
Ribback S, Cigliano A, Kroeger N, Pilo MG, Terracciano L, Burchardt M, Bannasch P, Calvisi DF, Dombrowski F (2015) PI3K / AKT / mTOR pathway plays a major pathogenetic role in glycogen accumulation and tumor development in renal distal tubules of rats and men. Oncotarget 6:13036–13048
Riordan NH, Ichim TE, Min W, Wang H, Solano F, Lara F, Alfaro M, Rodriguez JP, Harman RJ, Patel AN, Murphy MP, Lee RR, Minev B (2009) Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7:29–37
Rookmaaker MB, Verhaar MC, de Boer HC, Goldschmeding R, Joles JA, Koomans HA, Grone H, Rabelink TJ (2007) Met-RANTES reduces endothelial progenitor cell homing to activated ( glomerular ) endothelium in vitro and in vivo. Am J Physiol Renal Physiol 293:624–630
Shibuya M (2013) Endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19
Thadani JM, Marathe A, Vakodikar S, Kshatriya P, Modi D, Vyas R, Vyas B (2017) Treatment of type I diabetes using autologous adipose derived mesenchymal stem cells translated to insulin secreting islet like cell aggregates. J Case Reports 7:235–238
Wang S, Li Y, Zhao J, Zhang J, Huang Y (2013) Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant 19:538–546
Wang H, Qiu X, Ni P, Qiu X, Lin X, Wu W, Xie L, Lin L, Min J, Lai X, Chen Y, Ho G, Ma L (2014) Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int J Mol Med 33:263–270
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue multilineage cells from human adipose tissue. Tissue Eng 7:211–228
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayed, S.Y., Salem, S.I., Abdallah, A.N. et al. Clinicopathological studies on the use of laser-activated adipose-derived stromal vascular fraction in treatment of streptozotocin-induced diabetes in rats. Comp Clin Pathol 28, 1515–1526 (2019). https://doi.org/10.1007/s00580-019-03008-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03008-8