Abstract
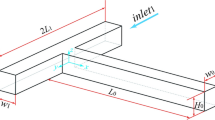
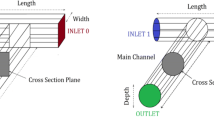
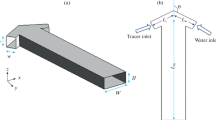
This study aims to investigate the micromixing performance of three basic types of spatial shaped micromixers. New configurations of T, Y, and oriented Y-spatial mixers were designed with change in the angles of the confluence and the outlet channel to achieve the efficient micromixing. These micromixers offer advantages that are not attainable with the typical types of these mixers. Experimental tests were carried out in the laminar flow regime and the mixing efficiency was evaluated using Villermaux/Dushman test reaction. The geometries of the channels were cylindrical with the length of 30 mm and the diameter of 800 μm. The experimental results show that the angle of outlet channel has a significant effect on the pressure drop and segregation index. Generally, the results reveal that at various feed flow rates the spatial shape of channels can lead to considerable improvement in micromixing performance. In all T, Y, and oriented Y-mixers, significant enhancement by increasing the confluence angle was also seen because the fluid elements were stretched and folded in the two inlet fluid interfaces. Furthermore, the micromixing time for the more efficient geometry of three shapes of microchannels was determined based on the incorporation model, which it was in the range of 0.001–0.1 s.









Similar content being viewed by others
Abbreviations
- A:
-
Light absorption
- Ci :
-
Concentration of tracer at time ti, mol L−1
- F:
-
Molar flux, mol s−1
- g(t):
-
Growth function of incorporation law
- [H+]0 :
-
Initial concentration of H+ ion, mol L−1
- I:
-
Ionic strength, mol L3
- Keq :
-
Equilibrium constant, L mol−1
- ki :
-
Kinetic constant
- Lo :
-
Length of outlet channel, m
- Lt :
-
Total length of inlet and outlet channels, m
- ΔP:
-
Pressure drop difference, Pa
- rj :
-
Net production rate of species j for the reaction, mol/m3 s
- R:
-
Flow rate ratio, dimensionless
- T:
-
Temperature, K
- t:
-
Time, s
- tm :
-
Characteristic micromixing time, s
- V:
-
Volume of fluid in the channel, m3
- Vacid :
-
Volume of acid at t, m3
- Vacid,0 :
-
Initial volume of acid, m3
- Y:
-
Selectivity of iodide, dimensionless
- YTS :
-
Selectivity of iodide for total segregation, dimensionless
- ε:
-
Specific power dissipation, W kg−1
- ρ:
-
Liquid density, kg m−3
- γ:
-
Specific weight, kg m−2 s−2
References
Alam A, Afzal A, Kim KY (2014) Mixing performance of a planar micromixer with circular obstructions in a curved microchannel. Chem Eng Res Des 92:423–434
Ansari MA, Kim KY, Anwar K, Kim SM (2010) A novel passive micromixer based on unbalanced splits and collisions of fluid streams. J Micromech Microeng 20:055007 (10 pp)
Aoki N, Mae K (2006) Effects of channel geometry on mixing performance of micromixers using collision of fluid segments. Chem Eng J 118:189–197
Aoki N, Umei R, Yoshida A, Mae K (2011) Design method for micromixers considering influence of channel confluence and bend on diffusion length. Chem Eng J 167:643–650
Aoki N, Fukuda T, Maeda N, Mae K (2013) Design of confluence and bend geometry for rapid mixing in microchannels. Chem Eng J 227:198–202
Asadi M, Xie G, Sunden B (2014) A review of heat transfer and pressure drop characteristics of single and two-phase microchannels. Int J Heat Mass Transfer 79:34–53
Baccar N, Kieffer R, Charcosset C (2009) Characterization of mixing in a hollow fibermembrane contactor by the iodide–iodate method: numerical simulations and experiments. Chem Eng J 148:512–524
Balan CM, Broboana D, Balan C (2010) Mixing process of immiscible fluids in microchannels. Int J Heat Fluid Flow 31:1125–1133
Commenge JM, Falk L (2011) Villermaux–Dushman protocol for experimental characterization of micromixers. Chem Eng Process 50:979–990
Ergin FG, Watz BB, Erglis K, Cebers A (2015) Time-resolved velocity measurements in a magnetic micromixer. Exp Therm Fluid Sci 67:6–13
Falk L, Commenge J-M (2010) Performance comparison of micromixers. Chem Eng Sci 65:405–411
Fang Y, Ye Y, Shen R, Zhu P, Guo R, Hu Y, Wu L (2012) Mixing enhancement by simple periodic geometric features in microchannels. Chem Eng J 187:306–310
Faryadi M, Rahimi M, Safari S, Moradi M (2014) Effect of high frequency ultrasound on micromixing efficiency in microchannels. Chem Eng Process 77:13–21
Fournier MC, Falk L, Villermaux J (1996) A new parallel competing reaction system for assessing micromixing efficiency-determination of micromixing time by a sample mixing model. Chem Eng Sci 51:5187–5192
Guichardon P, Falk L (2000) Characterisation of micromixing efficiency by the iodide-iodate reaction system (I) Experimental procedure. Chem Eng Sci 55:4233–4243
Hessel V, Lowe H, Schonfeld F (2005) Micromixers—a review on passive and active mixing principles. Chem Eng Sci 60:2479–2501
Hossain S, Ansari MA, Kim KY (2009) Evaluation of the mixing performance of three passive micromixers. Chem Eng J 150:492–501
Hsieh SS, Lin JW, Chen JH (2013) Mixing efficiency of Y-type micromixers with different angles. Int J Heat Fluid Flow 44:130–139
Jiao W, Liu Y, Qi G (2010) A new impinging stream–rotating packed bed reactor for improvement of micromixing iodide and iodate. Chem Eng J 157:168–173
Kanaris AG, Mouza AA (2011) Numerical investigation of the effect of geometrical parameters on the performance of a micro-reactor. Chem Eng Sci 66:5366–5373
Kashid M, Renken A, Minsker LK (2011) Mixing efficiency and energy consumption for five generic microchannel designs. Chem Eng J 167:436–443
Kockmann N, Kiefer T, Engler M, Woias P (2006) Convective mixing and chemical reactions in microchannels with high flow rates. Sens Actuat B. 117:495–508
Kolbl A, Kraut M, Schubert K (2008) The iodide iodate method to characterize micro structured mixing devices. AIChE J 54:639–645
Kuang A, Guangwen C, Lei S, Yang X, Liangliang Z, Jianfeng C (2009) Micromixing efficiency of viscous media in micro-channel reactor. Chin J Chem Eng 17:546–551
Monnier H, Wilhelm AM, Delmas H (1999) Influence of ultrasound on micromixing in a semi-batch reactor. Chem Eng Sci 54:2953–2961
Monnier H, Wilhelm AM, Delmas H (2000) Influence of ultrasound on micromixing in a semi-batch reactor. Chem Eng Sci 55:4009–4020
Nguyen NT, Wu Z (2005) Micromixers—a review. J Micromech Microeng 15:R1–R16
Nouri L, Legrand J, Benmalek N, Imerzoukene F, Yeddou AR, Halet F (2008) Characterization and comparison of the micromixing efficiency in torus and batch stirred reactors. Chem Eng J 142:78–86
Parvizian F, Rahimi M, Azimi N (2012) Macro- and micromixing studies on a high frequency continuous tubular sonoreactor. Chem Eng Process 57–58:8–15
Parvizian F, Rahimi M, Azimi N, Alsairafi AA (2014) CFD modeling of micromixing and velocity distribution in a 1.7-MHz tubular sonoreactor. Chem Eng Technol 37:1–11
Perez AC, Barrass S, Gavriilidis A (2010) Residence time distributions in microchannels: comparison between channels with herringbone structures and a rectangular channel. Chem Eng J 160:834–844
Rahimi M, Azimi N, Parvizian F (2013) Using microparticles to enhance micromixing in a high frequency continuous flow sonoreactor. Chem Eng Process 70:250–258
Rahimi M, Azimi N, Parvizian F, Alsairafi AA (2014) Computational fluid dynamics modeling of micromixing performance in presence of microparticles in a tubular sonoreactor. Comput Chem Eng 60:403–412
Shang X, Huang X, Yang C (2015) Mixing enhancement by the vortex in a microfluidic mixer with actuation. Exp Therm Fluid Sci 67:57–61
Steinke ME, Kandlikar SG (2006) Single-phase liquid friction factors in microchannels. Int J Therm Sci 45:1073–1083
Su Y, Chen G, Yuan Q (2011) Ideal micromixing performance in packed microchannels. Chem Eng Sci 66:2912–2919
Unadkat H, Nagy ZK, Rielly CD (2013) Investigation of turbulence modulation in solid–liquid suspensions using parallel competing reactions as probes for micro-mixing efficiency. Chem Eng Res Des 91:2179–2189
Wang L, Liu D, Wang X, Han X (2012a) Mixing enhancement of novel passive microfluidic mixers with cylindrical grooves. Chem Eng Sci 81:157–163
Wang W, Zhao S, Shao T, Zhang M, Jin Y, Cheng Y (2012b) Numerical study of mixing behavior with chemical reactions in micro-channels by a lattice Boltzmann method. Chem Eng Sci 84:148–154
Zhendong L, Yangcheng L, Jiawei W, Guangsheng L (2012) Mixing characterization and scaling-up analysis of asymmetrical T-shaped micromixer: experiment and CFD simulation. Chem Eng J 181–182:597–606
Author information
Authors and Affiliations
Corresponding author
Appendix: Kinetic of the Villermaux–Dushman reaction
Appendix: Kinetic of the Villermaux–Dushman reaction
The kinetics of the iodide-iodate chemical test reaction for characterizing micromixing efficiency was presented in literature. In summary, the reaction rate equations are as follows (Rahimi et al. 2014):
While k 1 = 1011 L mol−1 s−1
k2 is measured relative to ionic strength (I) of solution (Parvizian et al. 2012):
I is defined as a function of all ion concentrations (c) in the solution and their charge number (z) (Falk and Commenge 2010):
In the present work, the calculated value of I is 1.01393. The maximum absolute uncertainty of I is 0.0012. Therefore, the value of k2 according to Eq. (24) is equal to 1.26 × 107 mol L−1 s−1 (Falk and Commenge 2010; Rahimi et al. 2014).
at 25 °C:
The mole number of I2 can be calculated in terms of mass balance of iodine atoms and chemical equilibrium of reaction. The mass balance of reaction (3) expresses as follows (Jiao et al. 2010):
The equilibrium constant KB is a factor that is related to temperature, which follows the equation as (Kolbl et al. 2008; Jiao et al. 2010; Zhendong et al. 2012):
In this work, all the experiments were conducted at 25 °C, and then the value of KB was constant at 702 L/mol.
Rights and permissions
About this article
Cite this article
Rahimi, M., Azimi, N., Parsamogadam, M.A. et al. Mixing performance of T, Y, and oriented Y-micromixers with spatially arranged outlet channel: evaluation with Villermaux/Dushman test reaction. Microsyst Technol 23, 3117–3130 (2017). https://doi.org/10.1007/s00542-016-3118-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-016-3118-6