Abstract
Purpose
To evaluate the effects of sex on miRNA expression in the hippocampus after isoflurane anesthesia in a neonatal piglet model.
Methods
Six male and 6 female piglets, aged 3–5 days, were anesthetized with 2% isoflurane in room air for 3 h. Full physiologic monitoring was observed. Untreated animals (6 male, 6 female) served as controls. Expression of miRNAs in hippocampus was assessed.
Results
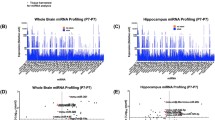
In controls, miRNA expression in the hippocampus was highly conserved between males and females. However, 17/326 displayed sex-dependent differences: 10 miRNAs were more highly expressed in males; 7 showed lower expression in males than females. Isoflurane was associated with changes in the expression of distinct subsets of miRNAs in both males and females. In females, 14/326 miRNAs were significantly changed (3 downregulated; 11 upregulated); in males, 17/326 miRNAs were changed (7 downregulated; 10 upregulated). There was no overlap in significantly changed miRNAs between isoflurane-exposed males and females.
Conclusions
In the neonatal piglet hippocampus, miRNA expression was highly conserved. There was no overlap in miRNA expression between isoflurane-exposed males and females, suggesting sex differences in isoflurane-induced miRNA expression. These results support the hypothesis that a clinically relevant exposure to isoflurane induces distinct miRNA signatures in the hippocampus of neonatal male and female piglets. Their functional relevance in anesthesia-induced neurotoxicity remains unknown, although changes in specific miRNAs may either contribute to or protect against anesthesia-induced neurotoxicity.






Similar content being viewed by others
References
Disma N, Mondardini MC, Terrando N, Absalom AR, Bilotta F. A systematic review of methodology applied during preclinical anesthetic neurotoxicity studies: important issues and lessons relevant to the design of future clinical research. Paediatr Anaesth. 2016;26(1):6–36.
FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2016. https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm. Accessed 1 July 2019.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
Gao FB. Context-dependent functions of specific microRNAs in neuronal development. Neural Dev. 2010;5:25.
Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79.
Barbato C, Ruberti F, Cogoni C. Searching for MIND: microRNAs in neurodegenerative diseases. J Biomed Biotechnol. 2009;2009:871313.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–83.
Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31(33):11748–55.
Lizarraga D, Huen K, Combs M, Escudero-Fung M, Eskenazi B, Holland N. miRNAs differentially expressed by next-generation sequencing in cord blood buffy coat samples of boys and girls. Epigenomics. 2016;8(12):1619–35.
Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78(Suppl 1):172–9.
Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci USA. 2011;108(28):11662–7.
Xie H, She GM, Wang C, Zhang LY, Liu CF. The gender difference in effect of sevoflurane exposure on cognitive function and hippocampus neuronal apoptosis in rats. Eur Rev Med Pharmacol Sci. 2015;19(4):647–57.
Gan TJ, Glass PS, Sigl J, Sebel P, Payne F, Rosow C, Embree P. Women emerge from general anesthesia with propofol/alfentanil/nitrous oxide faster than men. Anesthesiology. 1999;90(5):1283–7.
Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83.
Niblock MM, Luce CJ, Belliveau RA, Paterson DS, Kelly ML, Sleeper LA, Filiano JJ, Kinney HC. Comparative anatomical assessment of the piglet as a model for the developing human medullary serotonergic system. Brain Res Rev. 2005;50:169–83. https://doi.org/10.1016/j.brainresrev.2005.05.006.
Glauser EM. Advantages of piglets as experimental animals in pediatric research. Exp Med Surg. 1966;24(2):181–90.
Whitaker EE, Bissonnette B, Miller AD, Koppert TL, Tobias JD, Pierson CR, Christofi FL. A novel, clinically relevant use of a piglet model to study the effects of anesthetics on the developing brain. Clin Transl Med. 2016;5(1):2.
Guo L, Zhang Q, Ma X, Wang J, Liang T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci Rep. 2017;7:39812.
Li MM, Jiang T, Sun Z, Zhang Q, Tan CC, Yu JT, Tan L. Genome-wide microRNA expression profiles in hippocampus of rats with chronic temporal lobe epilepsy. Sci Rep. 2014;4:4734.
Twaroski D, Bosnjak ZJ, Bai X. MicroRNAs: new players in anesthetic-induced developmental neurotoxicity. Pharm Anal Acta. 2015;6:357.
Cao SE, Tian J, Chen S, Zhang X, Zhang Y. Role of miR-34c in ketamine-induced neurotoxicity in neonatal mice hippocampus. Cell Biol Int. 2015;39(2):164–8.
Lau P, de Strooper B. Dysregulated microRNAs in neurodegenerative disorders. Semin Cell Dev Biol. 2010;21(7):768–73.
Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J Neurosci. 2008;28(53):14341–6.
Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci USA. 2009;106(9):3507–12.
Twaroski DM, Yan Y, Olson JM, Bosnjak ZJ, Bai X. Down-regulation of microRNA-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology. 2014;121(4):786–800.
Chen Y, Zhang L, Teng H, Zhou H. MicroRNA 218 modulates PKC/AKT pathway to protect lidocaine-induced neurotoxicity in ganglia in vitro. Int J Clin Exp Pathol. 2017;10(5):5514–21.
Chen L, Wang X, Huang W, Ying T, Chen M, Cao J, Wang M. MicroRNA-137 and its downstream target LSD1 inversely regulate anesthetics-induced neurotoxicity in dorsal root ganglion neurons. Brain Res Bull. 2017;135:1–7.
Ye J, Zhang Z, Wang Y, Chen C, Xu X, Yu H, Peng M. Altered hippocampal microRNA expression profiles in neonatal rats caused by sevoflurane anesthesia: microRNA profiling and bioinformatics target analysis. Exp Ther Med. 2016;12(3):1299–310.
Shao D, Wu Z, Bai S, Fu G, Zou Z. The function of miRNA153 against isofluraneinduced neurotoxicity via Nrf2/ARE cytoprotection. Mol Med Rep. 2019;19(5):4001–100.
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–8.
Acknowledgements
We thank the Genomics Shared Resource at The Ohio State University Comprehensive Cancer Center (OSU CCC), Columbus, OH for conducting the Affymetrix GeneChip® miRNA 4.0 Array analyses. This work was supported in part by the OSU CCC and the National Institutes of Health Grant P30 CA16058.
Funding
This study was generously supported by departmental start-up funds [EW], a Nationwide Children’s Hospital, Internal Funds, Intramural Grant [EW], and the Ohio State University Center for Clinical and Translational Science Davis-Bremer Pre-K Award [EW]. This work was also supported in part by the OSU College of Medicine Roessler research scholarship [BW, JX]. EW is also supported by an NIH Institute for Child Health and Development, Loan Repayment Award, NIH LRP grant, funded by the NIH NICHD to investigate potential neurotoxicity mechanisms of anesthetics in a neonatal piglet model. FLC has received support from NIH R01 NIDDK Grants DK093499 and DK133943, as well as a Dean’s Bridge Grant from the OSU Office of Research. miRNA analysis was supported in part by NCI P30CA016058 Shared Resource Funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest declared.
Ethical approval
This study was approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC), protocol # 2014A00000018.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Whitaker, E.E., Wiemann, B.Z., Xia, J.C. et al. Distinct, sex-dependent miRNA signatures in piglet hippocampus induced by a clinically relevant isoflurane exposure: a pilot study. J Anesth 33, 670–679 (2019). https://doi.org/10.1007/s00540-019-02695-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-019-02695-5