Abstract
Purpose
To provide optimal conditions for neurophysiological monitoring and rapid awakening, remifentanil is commonly used during pediatric spinal surgery. However, remifentanil may induce hyperalgesia and increase postoperative opioid requirements. We evaluated the potential of methadone or magnesium to prevent remifentanil-induced hyperalgesia.
Methods
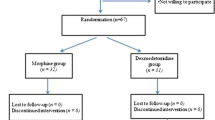
Using a prospective, randomized, blinded design, adolescents presenting for posterior spinal fusion to treat idiopathic scoliosis were assigned to receive desflurane with remifentanil alone (REMI), remifentanil + methadone (MET) (0.1 mg/kg IV over 15 min), or remifentanil + magnesium (MAG) (50 mg/kg bolus over 30 min followed by 10 mg/kg/h). Primary outcomes were opioid requirements and postoperative pain scores. Secondary outcomes included intraoperative anesthetic requirements, neurophysiological monitoring conditions, and emergence times.
Results
Data analysis included 60 patients. Total opioid requirement (hydromorphone) in the REMI group (received perioperatively and on the inpatient ward) was 0.34 ± 0.11 mg/kg compared to 0.26 ± 0.10 mg/kg in the MET group (95% confidence interval (CI) of difference: − 0.14, − 0.01; p = 0.035). The difference in opioid requirements between the REMI and MET group was related to intraoperative dosing (0.04 ± 0.02 mg/kg vs. 0.02 ± 0.01 mg/kg; 95% CI of difference: − 0.01, − 0.02; p = 0.003). No difference was noted in pain scores, and no differences were noted when comparing the REMI and MAG groups.
Conclusion
With the dosing regimens in the current study, the only benefit noted with methadone was a decrease in perioperative opioid requirements. However, given the potential for hyperalgesia with the intraoperative use of remifentanil, adjunctive use of methadone appears warranted.
Similar content being viewed by others
References
Padberg AM, Bridewell KH. Spinal cord monitoring: current state of the art. Orthop Clin North Am. 1999;30:407–33.
Intraoperative Neurophysiology Committee. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1990;40:1644–6.
Clapcih AJ, Emerson RG, Roye DP Jr, Xie H, Gallo EJ, Dowling KC, Ramnath B, Heyer EJ. The effects of propofol, small-dose isoflurane, and nitrous oxide on cortical somatosensory evoked potential and bispectral index monitoring in adolescents undergoing spinal fusion. Anesth Analg. 2004;99:1334–40.
Imani F, Jafarian A, Hassani V, Khan ZH. Propofol–alfentanil vs. propofol–remifentanil for posterior spinal fusion including wake-up test. Br J Anaesth. 2006;96:583–6.
Kim WH, Lee JJ, Lee SM, Park MN, Park SK, Seo DW, Chung IS. Comparison of motor-evoked potentials monitoring in response to transcranial electrical stimulation in subjects undergoing neurosurgery with partial vs no neuromuscular block. Br J Anaesth. 2013;110:567–76.
Martin DP, Bhalla T, Thung A, Rice J, Beebe A, Samora W, Klamar J, Tobias JD. Volatile agents or total intravenous anesthesia for neurophysiological monitoring during posterior spinal fusion in adolescents with idiopathic scoliosis. Spine. 2014;39:E1318–24.
Sammartino M, Garra R, Sbaraglia F, De Riso M, Continolo N. Remifentanil in children. Paediatr Anaesth. 2010;20:246–55.
Crawford MW, Hickey C, Zaarour C, Howard A, Naser B. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662–7.
Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance. Anesthesiology. 2000;93:409–17.
Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62:1266–80.
Sharma S, Balireddy RK, Vorenkamp KE, Durieux ME. Beyond opioid patient controlled analgesia: a systemic review of analgesia after major spine surgery. Reg Anesth Pain Med. 2012;37:79–98.
Zhao M, Joo DT. Enhancement of spinal n-methyl-d-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 2008;109:308–17.
Englehardt T, Zaarour C, Naser B, Pehora C, de Ruiter J, Howard A, Crawford MW. Intraoperative low dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesth Analg. 2008;107:1170–5.
McDonnell C, Zaarour C, Hull R, Thalayasingam P, Pehora C, Ahier J, Crawford MW. Pre-treatment with morphine does not prevent the development of remifentanil-induced hyperalgesia. Can J Anesth. 2008;55:813–18.
Elsharnouby NM, Elsharnouby MM. Magnesium sulphate as a technique of hypotensive anaesthesia. Br J Anaesth. 2006;96:727–31.
Altan A, Turgut N, Yildiz F, Türkmen A, Ustün H. Effects of magnesium sulphate and clonidine on propofol consumption, haemodynamics and postoperative recovery. Br J Anaesth. 2005;94:438–41.
Levaux Ch, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intraoperative magnesium sulphate on pain relief and patient comfort after major orthopaedic surgery. Anaesthesia. 2003;58:131–5.
Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulfate for postoperative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand. 2009;53:1088–91.
Na HS, Lee JH, Hwang JY, Ryu JH, Han SH, Jeon YT, Do SH. Effects of magnesium sulphate on intraoperative neuromuscular blocking agent requirements and postoperative analgesia in children with cerebral palsy. Br J Anaesth. 2010;104:344–50.
Stemland CJ, Witte J, Colquhoun DA, Durieux ME, Langman LJ, Balireddy R, Thammishetti S, Abel MF, Anderson BJ. The pharmacokinetics of methadone in adolescents undergoing posterior spinal fusion. Paediatr Anaesth. 2013;23:51–7.
Sharma A, Tallchief D, Blood J, Kim T, London A, Kharasch ED. Perioperative pharmacokinetics of methadone in adolescents. Anesthesiology. 2011;115:1153–61.
Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. 2011;112:218–23.
Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Remifentanil-acute opioid tolerance and opioid-induced hyperalgesia: a systematic review. Am J Ther. 2015;22:e62–74.
Perelló M, Artés D, Pascuets C, Esteban E, Ey Batlle AM. Prolonged perioperative low-dose ketamine does not improve short and long-term outcomes after pediatric idiopathic scoliosis surgery. Spine. 2017;42:E304–12.
Funding
This project was supported by The Clinical and Translational Intramural Funding Program through the Research Institute at Nationwide Children’s Hospital (Columbus, Ohio).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no other funding or conflict of interest to report.
Ethics
The study was approved by the Institutional Review Board at Nationwide Children’s Hospital (IRB13-00036) and registered at ClinicalTrails.gov (NCT01795495). An investigational new drug (IND) application for this study was approved by The Food and Drug Administration (FDA) prior to study initiation (IND117889).
Additional information
The content is solely the responsibility of the authors and does not necessarily represent the official views of Nationwide Children’s Hospital.
About this article
Cite this article
Martin, D.P., Samora, W.P., Beebe, A.C. et al. Analgesic effects of methadone and magnesium following posterior spinal fusion for idiopathic scoliosis in adolescents: a randomized controlled trial. J Anesth 32, 702–708 (2018). https://doi.org/10.1007/s00540-018-2541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-018-2541-5