Abstract
Objective
Children with repeated exposures to anesthesia at an early age are at an increased risk of cognitive impairment. Data in the literature link increased developmental depolarizing γ-aminobutyric acid (GABA) type A receptor (GABAAR) at younger age to neurodevelopmental disorders. Here we investigated the involvement of GABAergic signaling during development in mediating the adverse effects of repeated sevoflurane exposures.
Methods
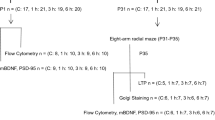
Sprague–Dawley male rats received repeated exposures to 3 % sevoflurane for 2 h daily for 3 consecutive days on postnatal days (P) 4, 5, and 6; maternally separated and unseparated rats served as controls. A subgroup of rats received three injections of the Na+–K+–2Cl− cotransporter inhibitor, bumetanide (1.82 mg/kg, intraperitoneally) 15 min prior to initiation of each sevoflurane exposure.
Results
The results showed that repeated neonatal sevoflurane exposures contribute to learning and memory impairment in the Morris water maze (MWM) at P60. The corticosterone level was significantly increased immediately after repeated neonatal sevoflurane exposures. Repeated neonatal sevoflurane exposures heightened the secretion of corticosterone in response to stress in P7 and P60 rats. Pretreatment of male rats prior to each sevoflurane exposure with bumetanide attenuated the corticosterone level immediately after repeated neonatal sevoflurane exposures, normalized endocrine response to stress at P7 and P60, and attenuated the sevoflurane-induced learning and memory impairment in the MWM.
Conclusion
These data suggested that the heightened stress response and cognitive impairment after repeated neonatal sevoflurane exposures might be linked to excessive GABAAR-mediated depolarization.





Similar content being viewed by others
References
Stratmann G. Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–9.
Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–75.
Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–86.
Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–23.
Zhang L-L, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol. 2006;95:2404–16.
Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13.
Ben-Ari Y, Gaiarsa J-L, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–84.
Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004;557:829–41.
Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600.
Glykys J, Dzhala VI, Kuchibhotla KV, Feng G, Kuner T, Augustine G, Bacskai BJ, Staley KJ. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63:657–72.
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–5.
Lee H, Chen CXQ, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21:2593–9.
Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+–Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36.
Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–9.
Mody I, Maguire J. The reciprocal regulation of stress hormones and GABAA receptors. Front Cell Neurosci. 2012;6:4.
Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology. 2014;231:3619–34.
Gao X-B, Van Den Pol AN. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J Neurophysiol. 2001;85:425–34.
Li Y, Xu Y, van den Pol AN. Reversed synaptic effects of hypocretin and NPY mediated by excitatory GABA-dependent synaptic activity in developing MCH neurons. J Neurophysiol. 2013;109:1571–8.
Mitev Y, Darwish M, Wolf S, Holsboer F, Almeida O, Patchev V. Gender differences in the regulation of 3α-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–9.
Morris RGM, Garrud P, Rawlins JNP, Okeefe J. Place navigation impaired in rats with hippocampal-lesions. Nature. 1982;297:681–3.
Macrí S, Mason GJ, Würbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci. 2004;20:1017–24.
Sousa VC, Vital J, Costenla AR, Batalha VL, Sebastião AM, Ribeiro JA, Lopes LV. Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol Aging. 2014;35:1680–5.
Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–38.
Willis J, Zhu W, Perez-Downes J, Tan S, Xu C, Seubert C, Gravenstein N, Martynyuk A. Propofol-induced electroencephalographic seizures in neonatal rats: the role of corticosteroids and γ-aminobutyric acid type A receptor-mediated excitation. Anesth Analg. 2015;120:433–9.
Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63.
Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86.
Wilder RT, Flick RP, Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804.
Acknowledgments
This work was supported by grants from the Health Department of Jiangsu Province (No. 90014002) and Yancheng Health Department (No. 2015037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
G. Liu and T. Zhu have contributed equally to this work.
About this article
Cite this article
Liu, G., Zhu, T., Zhang, A. et al. Heightened stress response and cognitive impairment after repeated neonatal sevoflurane exposures might be linked to excessive GABAAR-mediated depolarization. J Anesth 30, 834–841 (2016). https://doi.org/10.1007/s00540-016-2215-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-016-2215-0