Abstract
Background
Glioblastoma is an incurable disease with a poor prognosis. For caregivers of people with glioblastoma, the burden of care can be high. Patients often present with different clinical characteristics, which may impact caregiver burden in different ways. This study aimed to evaluate associations between patient clinical characteristics and caregiver burden/quality of life (QoL).
Methods
Caregiver–patient dyads were enrolled at 7 academic cancer centers in the United States. Eligible caregiver participants were self-reported as the primary caregiver of an adult living with glioblastoma and completed a caregiver burden survey. Eligible patients were age ≥ 18 years at glioblastoma diagnosis and alive when their respective caregiver entered the study, with the presence of cognitive dysfunction confirmed by the caregiver. Data were analyzed with descriptive statistics and multivariable analyses.
Results
The final cohort included 167 dyads. Poor patient performance status resulted in patient difficulty with mental tasks, more caregiving tasks, and increased caregiving time. Language problems were reported in patients with left-sided lesions. Patient confusion was negatively associated with all caregiver domains: emotional health, social health, general health, ability to work, confidence in finances, and overall QoL. Better caregiver QoL was observed in patients with frontal lobe lesions versus non-frontal lobe lesions.
Conclusion
This study reinforced that patient performance status is a critical clinical factor that significantly affects caregiver burden, caregiving tasks, and caregiver time. Additionally, patient confusion affects multiple facets of caregiver burden/QoL. These results could be used to support guided intervention for caregiver support, customized to the patient experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most aggressive central nervous system malignancy [36]. The disease is associated with a median overall survival of 15 months and a 5-year survival rate of < 5% [36]. Glioblastoma is incurable, so the treatment goal is typically preserving quality of life (QoL). Standard of care for newly diagnosed glioblastoma is surgery followed by combination radiation and temozolomide (TMZ) therapy [12, 26]. Most patients develop recurrence 6 to 9 months after primary treatment [20]. Factors for more favorable prognosis include O6-methylguanine-DNA methyltransferase promoter (MGMT) methylation and the presence of isocitrate dehydrogenase (IDH1) mutation, whereas increased age, poor patient performance status, increased number of lesions, and subtotal resection of tumor at surgery (compared with gross total resection) are associated with poor prognosis [8, 29, 32, 37, 40]. Cognitive dysfunction may be present at the time of glioblastoma diagnosis and generally worsens over time due to disease sequelae and/or treatment toxicity [17].
Poor prognosis, aggressive disease course, and cognitive dysfunction likely intensify caregiver burden; currently, however, there are limited data qualifying this association. Previous studies for evaluating caregiver burden in this patient group have been limited by use of non-specific survey instruments [11, 14, 22]. However, caregiver burden, for those caring for people with malignant glioma, has been shown to be significantly higher than for those caring for patients with other cancers [18]. A survey instrument is under development to evaluate burden and QoL in caregivers of patients with glioblastoma and cognitive dysfunction [3, 4]. This survey was used in the current study to evaluate associations between patient clinical characteristics and caregiver burden/QoL.
Materials and methods
Study design
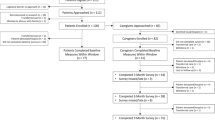
This study used a mixed-methods design, including parallel and cross-sectional comparisons with both a prospective component and a retrospective component. A national cohort of caregiver–patient dyads across 7 academic cancer centers in the United States were included in the study from April 2018 through June 2019. The 7 centers included 4 Oncology Research Information Exchange Network (ORIEN) [27] member sites (Huntsman Cancer Institute, Moffitt Cancer Center, the James Cancer Hospital at the Ohio State University, and University of Colorado Cancer Center) and 3 academic sites with expertise in glioblastoma (Duke University; University of California, Los Angeles; University of California, San Francisco). Each center obtained local scientific and institutional review board approval, recruited study participants, and securely transmitted de-identified data to the University of Utah for analysis. Caregivers and patients gave written informed consent to participate in the study; patients had given broad consent previously to have access to their medical records, which were used in this study.
Caregivers were surveyed once each with the new glioblastoma-specific caregiver burden instrument [3, 4] and the Caregiver Quality of Life Index-Cancer (CQOL-C) questionnaire [38]. The new glioblastoma-specific caregiver burden instrument is currently being developed, and is designed to assess the impact of each of the 7 domains of caregiver burden included in the survey scored from 1 to 10 [3, 4]. This survey has not yet been validated. The survey explored caregiver general burden, including emotional health, change in cognitive function of loved one with glioblastoma, information needs, caregiving responsibilities, caregiver’s health needs, and caregiver’s work and finances. These domains were evaluated with a focus on the impact of patients’ cognitive dysfunction on caregiver burden. CQOL-C is a validated, 35-item questionnaire that evaluates facets of a caregiver’s well-being and determines a final score on a scale of 0 to 100; a higher score represents better QoL [38]. The CQOL-C questionnaire has previously been shown to correlate with the instrument used in this analysis [3, 4]. At the time that a caregiver completed the surveys, clinical data of their respective patient currently living with glioblastoma were abstracted from medical records via an automated data pull of discrete variables and/or review of clinical notes facilitated by a standardized case report form built in REDCap® [28].
Eligibility criteria
Eligible caregiver participants were aged ≥ 18 years who self-identified as the primary caregiver of an individual living with glioblastoma; acknowledged fifth-grade or higher literacy proficiency in English at screening; indicated perceiving cognitive dysfunction in the patient they cared for; and had not participated in the pilot study. Patients with glioblastoma were eligible if they were alive at the time their caregiver enrolled in the study; were aged ≥ 18 years when diagnosed with glioblastoma; had confirmed diagnosis by two International Classification of Diseases (ICD)-9 (191.7, 191.8, 191.9) or ICD-10 codes (V10.85, 71.9), physician documentation, or ICD-O-3 or pathology-confirmed diagnosis; and had two or more healthcare encounters with one encounter at least 30 days from index date (date of diagnosis of glioblastoma).
Data collection
At each center, a research coordinator approached caregivers accompanying their patient to a clinic visit. Caregivers who consented to participate completed surveys electronically via Qualtrics® or on paper. Caregivers who started the survey electronically in Qualtrics completed their survey using a unique link that allowed them to access their incomplete survey electronically. Caregivers who started the survey in paper format completed the survey on paper and arranged with the study coordinator to return the survey in person or by mail.
Statistical analysis
Caregiver demographics and patient demographic and clinical characteristics were summarized using descriptive statistics. Patients were additionally summarized by subgroups by tumor MGMT methylation status and Karnofsky performance status scores. Patients were stratified by tumor MGMT status as it is a known prognostic factor that is prevalent in approximately 50% of people with glioblastoma. This allowed the assessment of any difference in methylated/unmethylated MGMT status on patient characteristics. Comparisons of patient characteristics between patient subgroups were evaluated using tests of association, including Chi square, Fisher’s exact, Wilcoxon rank-sum, analysis of variance, and Student’s t test, as appropriate. The Karnofsky performance status rubric defines patients with Karnofsky performance status ≥ 80 as able to carry on normal activity with no special care needed. Patients with a score of 80 have some signs or symptoms of disease, and those with 90 and 100 exhibit minor and no signs of disease, respectively. Predictive models for caregiver burden (defined as the impact of each of the 7 domains of caregiver burden included in the survey) were built using a backward elimination variable selection method. Given that the purpose of this model is to identify risk factors for increased caregiver burden in the glioblastoma population, variable selection was conducted based on a two-part approach. In step 1, a comprehensive list of demographic, clinical, and caregiver-reported variables was evaluated in a univariable model for association with caregiver burden. In step 2, a backward elimination process was conducted for all variables that showed an unadjusted association with caregiver burden in step 1 (i.e., variables that met a strict criterion for significance [p < 0.05]). To decrease the risk of false-positive associations, variables that had a p value > 0.05 were removed from the multivariable model one-by-one until the model included only significant risk factors for caregiver burden. STATA version 14.2 was used for statistical analysis.
Results
Patient demographics
The final cohort included 167 dyads recruited from 7 academic cancer sites between November 2018 and June 2019. These 167 dyads represent a subgroup of 185 dyads originally recruited, for which results on caregiver perceptions of cognitive dysfunction have previously been reported [3, 4]. There were 18 caregivers who completed the survey for which consent by the patient they cared for was not obtained. The mean (± standard deviation [SD]) age of patients at diagnosis was 57 (± 12) years, and the majority of patients were white (87%) and male (62%) (Table 1). Ten percent of patients had multifocal lesions and 60% had baseline Karnofsky performance status scores of 80–100. No significant differences in demographic features were observed for patients with methylated MGMT (mMGMT) versus unmethylated MGMT (uMGMT) tumor status. Overall, 23 patients (14%) had an IDH1 mutation–positive tumor. More patients with mMGMT versus uMGMT tumor status had IDH1 mutation–positive tumors (n = 13 vs 5, p = 0.05) (Table 1). Five patients (3%) had tumors with concurrent alterations in TERT and IDH1. This study did not distinguish between primary and secondary glioblastoma. At the time of survey completion, 44% of caregivers reported that patients were receiving treatment for initial glioblastoma diagnosis, 38% reported that patients were receiving treatment for glioblastoma recurrence, 15% reported that patients were not receiving treatment, and 2% reported that patients were receiving hospice/symptom management, respectively (patient treatment status was unknown for 1%).
Caregiver demographics
Caregivers were predominantly white (82%) and female (63%), and had a mean (± SD) age of 58 (± 12) years. The majority had attended college/graduate school (77%) and 36% reported household incomes > $100,000. Before glioblastoma diagnosis, 59% of caregivers were employed; at the time of enrollment, 44% of caregivers were employed. Many were patients’ spouses (84%). Forty-seven percent of caregivers had provided care for 6 to 23 months.
Treatment patterns
TMZ monotherapy was the most common first-line systemic treatment (89% of patients). Few patients received combination systemic therapy as initial treatment (Supplementary Table 1).
Cognitive dysfunction
When asked about the impact of cognitive dysfunction symptoms, caregivers ranked memory problems, changes in personality/mood, and language problems in their patient as the most impactful to them daily (Fig. 1).
Caregivers ranked memory problems, confusion, and problems completing mental tasks as the 3 most common cognitive dysfunction symptoms observed in patients in the last 14 days. More caregivers of patients with mMGMT tumor status, rather than uMGMT tumor status, reported their patient having problems completing familiar tasks (67% vs 45%, p = 0.049) and problems completing mental tasks (82% vs 61%, p = 0.04) (Table 2).
Caregiver tasks and time
Caregiving tasks, including “mobility and transportation,” “meal preparation,” “bathroom needs,” “medication organization/administration,” “other household tasks (e.g., cleaning, grocery shopping),” and “other caregiving activities,” were evaluated for association with patient clinical characteristics. More caregivers of patients with low versus high Karnofsky performance status reported performing tasks related to “mobility and transportation” (87% for Karnofsky performance status ≤ 80 vs 68% for Karnofsky performance status 90–100, p < 0.01) and “bathroom needs” (44% for Karnofsky performance status ≤ 80 vs 21% for Karnofsky performance status 90–100, p < 0.01). Similarly, more caregivers of patients with a lesion involving the frontal lobe indicated performing tasks related to “bathroom needs” compared with caregivers of patients with a lesion not in the frontal lobe (43% vs 28%, p = 0.05). More caregivers of patients with concurrent mMGMT and IDH1 mutation–positive tumor status reported performing tasks related to “medication organization/administration” compared with patients with concurrent uMGMT and IDH1 mutant tumor status (77% vs 20%, p = 0.047). No associations were seen between caregiving tasks and surgery at initial diagnosis, number or site of lesions, TERT and IDH1 mutation status, IDH1 mutation status alone, or length of time as caregiver (results not shown). Patients who received partial resections were significantly more likely to require assistance using the bathroom (p < 0.01). No other associations were found between type of surgery and caregiver tasks.
Caregiving time and patient clinical characteristics were also evaluated. Caregivers were asked, “On average, how many hours each week do you spend directly providing care as a result of the glioblastoma diagnosis? Examples include preparing meals, grooming, lifting, and administering medication.” Time was categorized as 1 to 3, 4 to 6, 7 to 9, 10 to 12, and > 12 h weekly. More caregivers of patients with lower baseline Karnofsky performance status compared with higher Karnofsky performance status devoted > 12 h weekly for providing care (58% for Karnofsky performance status ≤ 80 vs 29% for Karnofsky performance status 90–100, p < 0.01). No associations were seen between caregiving time and glioblastoma recurrence, surgery at initial diagnosis, extent of surgery, number/location/side of lesion, or tumor genomics (TERT and IDH1 mutation status, MGMT and IDH1 mutation status, IDH1 mutation status alone) (results not shown).
Caregiver QoL
The overall median CQOL-C score was 81.5 (interquartile range, 68–98). Median CQOL-C score was higher among caregivers of patients with a tumor involving the frontal lobe compared with caregivers of patients with non-frontal lobe lesions (89 vs 78.5, p = 0.04). No significant associations were seen between caregiver QoL and glioblastoma recurrence, baseline Karnofsky performance status, surgery at diagnosis, number or site of lesions, tumor MGMT status, TERT and IDH1 mutation status, MGMT methylation and IDH1 mutation status, or IDH1 mutation status alone.
Predictive factors for caregiver burden
Different domains of caregiver burden were examined with univariable and multivariable models, including clinical and nonclinical variables. Multivariable analysis showed that patient changes in personality/mood versus no changes (p = 0.01), patient confusion (yes vs no, p < 0.01), and caregiver task of “medication administration” (yes vs no, p = 0.02) were independent predictors of poorer emotional health of the caregiver (Table 3). Patient sex (male vs female, p < 0.01), time commitment > 12 h weekly versus < 12 h weekly (p = 0.01), patient confusion (yes vs no, p < 0.01), and “transportation” (yes vs no, p = 0.02) were independent contributors to predicting poorer caregiver social health. Similarly, patient sex (male vs female; p < 0.01), time commitment > 12 h weekly versus < 12 h weekly (p = 0.01), and patient confusion (yes vs no, p < 0.01) were independent contributors to predicting poorer caregiver general health (Table 3). Multivariable analysis showed that independent predictors of caregiver inability to work included patient confusion (yes vs no, p = 0.01), patient poor judgment (yes vs no, p = 0.01), and providing medication (yes vs no, p < 0.01) (Table 4). Independent contributors to poor confidence in finances, as assessed by the caregiver, included patient sex (male vs female, p = 0.03), older caregiver age (p < 0.01), patient confusion (yes vs no, p < 0.01), and “bathroom needs” (yes vs no, p < 0.01). Lastly, patient confusion and poor judgment (yes vs no, both p < 0.01) were significantly associated with poor caregiver overall QoL (Table 4).
Discussion
This study used a novel survey explicitly designed to measure caregiver burden in those caring for patients with glioblastoma. Patient clinical factors affecting caregiver QoL were also assessed. Patients in this study were representative of the general glioblastoma patient population, with a predominance of males (1.6 × females) and initial presentation with a solitary lesion [31, 35]. Median age at diagnosis (59.2 years) was younger than in other reports (64 years) [35].
More than 90% of patients received guideline-supported first-line treatment. The majority of patients in this study had a good baseline performance status, with 85% of patients with a known Karnofsky performance status scoring > 80. Distributions of tumor MGMT status and EGFR, TERT, and IDH1 genomic alterations observed in the current study are consistent with those reported in the current literature [1, 5, 7, 10]. Methylation of MGMT in glioblastoma tumors occurs in approximately half of patients and is considered an independent prognostic marker for survival, and is predictive of response to TMZ [16, 21, 34, 39]. Among patients in this study with known tumor MGMT status, 51% had mMGMT.
Characteristics of caregivers of patients with glioblastoma in this study were mostly similar to those of other cancer caregivers; most caregivers were female and related to the patient, but they were older than the average cancer caregiver by 5 years and had achieved a higher level of education [23, 30]. Patient clinical characteristics influenced manifestations of cognitive dysfunction and caregiver experience in this study. Problems completing familiar tasks and problems completing mental tasks were associated with mMGMT tumor status. Lower baseline Karnofsky performance status was associated with difficulties completing mental tasks and poor judgment in the current study. These symptoms, such as memory loss, exemplify executive functions typically controlled by the brain’s frontal lobe [19]. However, in the current study, significantly more caregivers of patients with a lesion outside the frontal lobe reported that their patient had memory problems. In addition, memory problems were considered the most common and most impactful cognitive dysfunction symptom for caregivers on a daily basis. These results illustrate the complexity of neuro-oncological manifestations of glioblastomas as well as the caregiver experience.
Analysis of caregiving tasks and caregiver time demonstrated that significantly more time was required for “mobility and transportation” and “bathroom needs” for patients with lower baseline Karnofsky performance status. This finding is consistent with augmented caregiving typically needed for individuals with reduced functioning, even when comparing small differences in Karnofsky performance status scores. In this study, Karnofsky performance status scores were recorded at initial diagnosis. We did not evaluate if this decline was driven by disease alone and/or treatment toxicity, and the Karnofsky performance status score of the patient at the time of the caregiver survey was not recorded.
In this study, caregiver QoL was unaffected by the patient’s clinical characteristics except for the location of lesion, with a higher CQOL-C score significantly associated with tumor involving the frontal lobe. Since cognitive functions are primarily controlled by the frontal lobe, surgical excision of lesions from this area may produce more obvious improvements and higher perceived benefit, which could have affected the caregiver’s QoL.
The predictive models examining facets of caregivers’ health, ability to work, confidence in finances, and overall QoL illustrate the complexity of the caregiver experience. A common theme was the impact of patient confusion, which was an independent contributor to all these domains, although source of patient confusion was not evaluated. Nevertheless, future caregiver interventions should address this symptom. Caregivers could benefit from education about the development or worsening of confusion and receiving advice on practical techniques to manage situations where it plays a large role, especially if there is a safety concern. They also should be urged to initiate advanced care planning before confusion prohibits patient decision-making [13]. Caregivers could be encouraged to track details about their patient’s confusion to help determine its cause. For example, increased confusion may be seen after treatment with chemotherapy, radiation therapy, or steroids [9]. This information would be helpful toward development of future treatments for glioblastoma, or new modalities or modification of current therapies, that would enable effective control of disease with minimal neurological adverse effects.
A key strength of this study is that caregiver burden/QoL was evaluated against respective patient clinical characteristics across a national cohort [3, 4]. Collaborating sites in this cohort are specialized cancer centers with expertise in glioblastoma. Inclusion of living patients with glioblastoma also strengthened this study by minimizing caregiver recall bias. However, this study has several limitations. Recall bias may exist despite attempts to minimize this by enrolling only caregivers of living patients and asking about cognitive dysfunction symptoms in the last 14 days. In addition, “better-faring patient” bias similar to survival bias may exist. Multiple questions in the glioblastoma survey used a Likert scale [3, 4]; although this is a convenient and easy-to-understand measure, it assumes an even interval between measuring points. As previously noted, the new glioblastoma-specific caregiver burden instrument employed in this study is currently being developed and is not yet validated; however, its use in this study serves as an important resource in the ongoing validation process of this novel instrument. Results from this study come from a one-time survey administration and do not reflect the dynamic process of caregiving [24]. Some caregivers did not answer all the survey questions, leading to some missing data. However, questions had a random distribution of missing data with the majority of questions having missing responses from < 10% of caregivers. In those questions missing > 10% of responses, questions were skipped by some caregivers as some questions were not applicable to the whole group, i.e., a question about work skipped by a caregiver who does not work. Patient data were collected from medical records and are thus subject to the inherent biases of retrospective studies, such as missing or incomplete data. Due to the lack of previous research on caregiving burden in the glioblastoma population, the variable selection for these models was largely exploratory. Since the p values assume that each variable was pre-specified [33], the models created should be verified in future studies. The caregivers included in the survey were predominantly white and well educated and reported a very good income due to the location of the study centers involved in the survey development. As a result, we acknowledge that selection bias may be present. Finally, caregiver evaluations, including evaluations of the patients’ cognitive function, in this study were subjective, which may have led to potential bias in answers to survey questions.
Conclusion
The results of this study have significant implications for clinical practice. Previously, no instrument had been available that specifically focused on caregiver burden, for caregivers of patients with glioblastoma. This study demonstrated differences in the impact of patient baseline function on caregivers, the prevalence of patient confusion on multiple areas of caregiver life and burden, and the overall idea that not all glioblastoma caregiver–patient dyads are alike. These could lead to close collaboration among the patient, the caregiver, and the oncology multidisciplinary team to support caregivers who could benefit from interventions tailored to their individual circumstances. Early identification of specific caregiver domains affected by caregiving could be the first step in triggering an intervention by the multidisciplinary team. New caregivers overwhelmed by their new role could be encouraged to attend supportive care sessions that review practical strategies to help with daily activities of caregiving (nurse), including medication organization (pharmacist), transportation assistance (social work), creating a care network (social work), and optimizing home health services (nurse), all toward avoiding caregiver burnout. Veteran caregivers are more likely to experience caregiver burnout, and many caregivers develop mental health problems, including anxiety and depression [15, 25]; caregivers for patients with brain cancer experience lower levels of mental health than those in other cancers [2]. As such, caregivers would benefit from supportive care sessions aimed at reviewing self-care, normalization of feelings, and finding an appropriate patient–caregiver time balance, as well as support from a psychologist or a psychiatrist for assistance with mental health conditions. From a patient’s perspective, shared decision-making can provide a central role for the patient in the decision-making process through open communication, which could also help the caregiver provide optimal care to the patient [6].
This study used a new instrument that is being developed to assess how caregiver burden is affected by the patient’s clinical factors. Re-evaluation of this survey over the course of the disease would allow future research to investigate the dynamic process of caregiving. The variables included in the models as well as the reliability of the results should be verified in future studies to improve the measurement of burden among caregivers of patients with glioblastoma. In addition, evaluating caregiver QoL by geography, such as community cancer centers in rural areas, may highlight disparities in caregiver burden.
Data availability
Not applicable.
Code availability
STATA V15.1 was used for all analyses.
Change history
18 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00520-021-06691-y
References
An Z, Aksoy O, Zheng T, Fan QW, Weiss WA (2018) Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene 37:1561–1575
Aoun SM, Deas K, Howting D, Lee G (2015) Exploring the support needs of family caregivers of patients with brain cancer using the CSNAT: a comparative study with other cancer groups. PLoS One 10:e0145106
Au T, Bauer H, Ma J, Nelson R, Watanabe A, Singh P, Korytowsky B, Wilson E, Willmarth N, Cohen A, Colman H, Stenehjem D, Brixner D (2017) Hout-19. Development of a novel burden survey for caregivers of glioblastoma multiforme (GBM) patients. Neuro-Oncology 19:vi110–vi110
Au TH, Bauer H, Menon J, Willis C, Iacob E, Ma J, Watanabe A, Nelson R, Korytowsky B, Singh P, Marshall A, Willmarth N, Nghiemphu PL, Dovek L, Peters KB, Balajonda B, Cohen AL, Colman H, Stenehjem DD, Brixner DI (2018) Impact of glioblastoma (GBM)-related cognitive dysfunction (CD) on caregiver burden: preliminary results from multi-site study in the U.S. J Clin Oncol 36:16–16
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477
Brom L, De Snoo-Trimp JC, Onwuteaka-Philipsen BD, Widdershoven GAM, Stiggelbout AM, Pasman HRW (2017) Challenges in shared decision making in advanced cancer care: a qualitative longitudinal observational and interview study. Health Expect 20:69–84
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Chen JR, Yao Y, Xu HZ, Qin ZY (2016) Isocitrate dehydrogenase (IDH)1/2 mutations as prognostic markers in patients with glioblastomas. Medicine (Baltimore) 95:e2583
Coomans MB, van der Linden SD, Gehring K, Taphoorn MJB (2019) Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol 31:540–547
Crespo I, Vital AL, Gonzalez-Tablas M, Patino Mdel C, Otero A, Lopes MC, de Oliveira C, Domingues P, Orfao A, Tabernero MD (2015) Molecular and genomic alterations in glioblastoma multiforme. Am J Pathol 185:1820–1833
Deatrick JA, Hobbie W, Ogle S, Fisher MJ, Barakat L, Hardie T, Reilly M, Li Y, Ginsberg JP (2014) Competence in caregivers of adolescent and young adult childhood brain tumor survivors. Health Psychol 33:1103–1112
Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, Caeiro C (2017) Current standards of care in glioblastoma therapy. In: De Vleeschouwer S (ed) Glioblastoma. Codon Publications, Brisbane, pp 197–242
Fritz L, Dirven L, Reijneveld JC, Koekkoek JAF, Stiggelbout AM, Pasman HR, Taphoorn MJ (2016) Advance care planning in glioblastoma patients. Cancers (Basel) 8:102
Goebel S, von Harscher M, Mehdorn HM (2011) Comorbid mental disorders and psychosocial distress in patients with brain tumours and their spouses in the early treatment phase. Support Care Cancer 19:1797–1805
Grov EK, Dahl AA, Moum T, Fosså SD (2005) Anxiety, depression, and quality of life in caregivers of patients with cancer in late palliative phase. Ann Oncol 16:1185–1191
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Hilverda K, Bosma I, Heimans JJ, Postma TJ, Vandertop WP, Slotman BJ, Buter J, Reijneveld JC, Klein M (2010) Cognitive functioning in glioblastoma patients during radiotherapy and temozolomide treatment: initial findings. J Neurooncol 97:89–94
Jacobs DI, Kumthekar P, Stell BV, Grimm SA, Rademaker AW, Rice L, Chandler JP, Muro K, Marymont M, Helenowski IB, Wagner LI, Raizer JJ (2014) Concordance of patient and caregiver reports in evaluating quality of life in patients with malignant gliomas and an assessment of caregiver burden. Neurooncol Pract 1:47–54
Lee J, Hall CWR (2019) The impact of gliomas on cognition and capacity. J Am Acad Psychiatry Law 47:350–359
Mallick S, Benson R, Hakim A, Rath GK (2016) Management of glioblastoma after recurrence: a changing paradigm. J Egypt Natl Canc Inst 28:199–210
Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–926
Milbury K, Mallaiah S, Mahajan A, Armstrong T, Weathers SP, Moss KE, Goktepe N, Spelman A, Cohen L (2018) Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integr Cancer Ther 17:332–336
National Alliance for Caregiving (2016) Cancer caregiving in the U.S.: an intense, episodic, and challenging care experience. https://www.caregiving.org/wp-content/uploads/2020/05/CancerCaregivingReport_FINAL_June-17-2016.pdf. Accessed May 2020
Nijboer C, Triemstra M, Tempelaar R, Mulder M, Sanderman R, van den Bos GA (2000) Patterns of caregiver experiences among partners of cancer patients. Gerontologist 40:738–746
Nipp RD, El-Jawahri A, Fishbein JN, Gallagher ER, Stagl JM, Park ER, Jackson VA, Pirl WF, Greer JA, Temel JS (2016) Factors associated with depression and anxiety symptoms in family caregivers of patients with incurable cancer. Ann Oncol 27:1607–1612
Niranjan A, Kano H, Iyer A, Kondziolka D, Flickinger JC, Lunsford LD (2015) Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J Neurosurg 122:757–765
ORIEN (2020) Oncology Research Information Exchange Network. https://www.oriencancer.org/. Accessed May 2020
Patridge EF, Bardyn TP (2018) Research Electronic Data Capture (REDCap). J Med Libr Assoc 106:142–144
Scott JG, Chao ST, Suh JH, Cooper A, Reiner AS, Nayak L, Panageas K, Abrey LE, Iwamoto FM (2010) Prognostic factors for glioblastoma (GBM) in individuals age 70 and older: a study of 446 patients from two tertiary centers. J Clin Oncol 28:2034
Sharma N, Chakrabarti S, Grover S (2016) Gender differences in caregiving among family - caregivers of people with mental illnesses. World J Psychiatry 6:7–17
Singh G, Mehrotra A, Sardhara J, Das KK, Jamdar J, Pal L, Srivastava AK, Sahu RN, Jaiswal AK, Behari S (2015) Multiple glioblastomas: are they different from their solitary counterparts? Asian J Neurosurg 10:266–271
Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A (2012) Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg 114:840–845
Steyerberg EW (2009) Clinical prediction models: a practical approach to development, validation, and updating. Springer, New York
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Tamimi AF, Juweid M (2017) Epidemiology and outcome of glioblastoma. In: De Vleeschouwer S (ed) Glioblastoma, Chapter 8. Codon Publications, Brisbane
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 23:1985–1996
Wang XW, Ciccarino P, Rossetto M, Boisselier B, Marie Y, Desestret V, Gleize V, Mokhtari K, Sanson M, Labussiere M (2014) IDH mutations: genotype-phenotype correlation and prognostic impact. Biomed Res Int 2014:540236
Weitzner MA, Jacobsen PB, Wagner H Jr, Friedland J, Cox C (1999) The Caregiver Quality of Life Index–Cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Qual Life Res 8:55–63
Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A, Plass C, Hegi M, Platten M, Reifenberger G (2014) MGMT testing–the challenges for biomarker-based glioma treatment. Nat Rev Neurol 10:372–385
Zou P, Xu H, Chen P, Yan Q, Zhao L, Zhao P, Gu A (2013) IDH1/IDH2 mutations define the prognosis and molecular profiles of patients with gliomas: a meta-analysis. PLoS One 8:e68782
Acknowledgements
The authors received writing/editorial support in the preparation of this manuscript provided by Barrie Anthony, PhD, CMPP, of Evidence Scientific Solutions Inc., funded by Bristol Myers Squibb. We thank the Oncology Research Information Exchange Network (ORIEN) for support in recruitment of patients at 4 sites. We also thank Mikaela Larson at Huntsman Cancer Institute for her assistance with ORIEN sites. Lastly, we owe gratitude to the caregivers and patients who participated in this study.
Funding
This study was funded by Bristol Myers Squibb. The Total Cancer Care Protocol implementation at Huntsman Cancer Institute, Utah, was supported by the Huntsman Cancer Foundation.
Author information
Authors and Affiliations
Contributions
Concept and design—Trang H. Au, Connor Willis, Jyothi Menon, Junjie Ma, Nicole Willmarth, Prianka Singh, Alexander Marshall, Beata Korytowsky, David Stenehjem, Diana Brixner.
Collection and assembly of data—Trang H. Au, Connor Willis, Jyothi Menon, Maija Reblin, Katherine B. Peters, Phioanh Leia Nghiemphu, Jennie Taylor, Howard Colman, Adam L. Cohen, D. Ryan Ormond, Arnab Chakravarti, David Stenehjem, Diana Brixner.
Data analysis and interpretation—Trang H. Au, Connor Willis, Alexandre H. Watanabe, Alexander Marshall, Beata Korytowsky, David Stenehjem, Diana Brixner.
Manuscript writing—all authors.
Final approval of manuscript—all authors.
Accountable for all aspects of the work—all authors.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the institutional review boards of all study centers involved in the study.
Consent to participate
Caregivers and patients gave written informed consent to participate in the study; patients had given broad consent previously to have access to their medical records, which were used in this study.
Consent for publication
All authors consented to publish this paper.
Conflict of interest
Alexandre H. Watanabe, Nicole Willmarth, and Adam L. Cohen have no conflicts of interest.
Maija Reblin has received grants from Bristol Myers Squibb.
Phioanh Leia Nghiemphu has received grants from Genentech and Novartis.
Junjie Ma was an employee of the University of Utah at the time this study was conducted, is currently employed by Amgen Inc., and has received grants from Bristol Myers Squibb.
Jyothi Menon was an employee of the University of Utah at the time this study was conducted, and has received grants from Bristol Myers Squibb.
D. Ryan Ormond has received research funding from Agios, the American Cancer Society, American Heart Association, and Medtronic and is currently employed by Amgen Inc.
Katherine B. Peters received grant/research support from Bristol Myers Squibb.
Jennie W. Taylor received grant support from AbbVie, Agios, and Bristol Myers Squibb.
Howard Coleman reports personal fees from Adastra Pharmaceuticals, Bayer, Best Doctors/Teladoc, Forma Therapeutics, Karyopharm Therapeutics, Orbus, and Private Health.
Beata Korytowsky was an employee and shareholder of Bristol Myers Squibb at the time of study.
Alexander Marshall and Prianka Singh are employees of Bristol Myers Squibb.
David Stenehjem reports grants from Bristol Myers Squibb, and personal fees from Bristol Myers Squibb, Dracen Pharmaceuticals, Iterion Pharmaceuticals, Molecular Templates, and Salarius Pharmaceuticals.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Au, T.H., Willis, C., Reblin, M. et al. Caregiver burden by treatment and clinical characteristics of patients with glioblastoma. Support Care Cancer 30, 1365–1375 (2022). https://doi.org/10.1007/s00520-021-06514-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06514-0