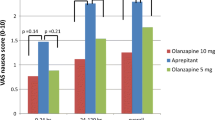
Abstract
Purpose
Olanzapine is effective in chemotherapy-induced nausea and vomiting (CINV). In patients receiving highly emetogenic chemotherapy (HEC), its efficacy was reported as rescue therapy for breakthrough emesis refractory to triplet therapy (palonosetron, aprepitant, and dexamethasone). However, its preventive effects with triplet therapy for CINV are unknown. This study aimed to investigate efficacy and safety of preventive use of olanzapine with triplet therapy for CINV of HEC.
Methods
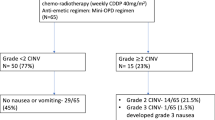
This study is a prospective multicenter study conducted by Kansai Clinical Oncology Group. Forty chemo-naïve gynecological cancer patients receiving HEC with cisplatin (≥50 mg/m2) were enrolled. Oral olanzapine (5 mg) was administered with triplet therapy a day prior to cisplatin administration and on days 1–5. The primary endpoint was complete response (no vomiting and no rescue) rate for the overall phase (0–120 h post-chemotherapy). Secondary endpoints were complete response rate for acute phase (0–24 h post-chemotherapy) and delayed phase (24–120 h post-chemotherapy) and complete control (no vomiting, no rescue, and no significant nausea) rate and total control (no vomiting, no rescue, and no nausea) rate for each phase. These endpoints were evaluated during the first cycle of chemotherapy.
Results
Complete response rates for acute, delayed, and overall phases were 97.5, 95.0, and 92.5 %, respectively. Complete control rates were 92.5, 87.5, and 82.5 %, respectively. Total control rates were 87.5, 67.5, and 67.5 %, respectively. There were no grade 3 or 4 adverse events.
Conclusions
Preventive use of olanzapine combined with triplet therapy gives better results than those from previously reported studies of triplet therapy.
Similar content being viewed by others
References
Bloechl-Daum B, Deuson RR, Mavros P, et al. (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. Clin Oncol 24:4472–4478
MASCC/ESMO Antiemetic Guideline 2013. http://www.mascc.org/assets/documents/mascc_guidelines_english_2013.pdf Accessed 20 Mar. 2015.
Basch E, Prestrud AA, Hesketh PJ, et al. (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
National Comprehensive Cancer Network (2014) NCCN clinical practice guidelines in oncology: antiemesis, Version 1. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf Accessed 20 Mar. 2015.
Japan Society of Clinical Oncology (2014) Guidelines for the proper use of antiemetics, version 1.2. Kanehara, Tokyo.
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098
Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17:1000–1006
Campos D, Pereira JR, Reinhardt RR, et al. (2001) Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol 19:1759–1767
Hesketh PJ, Grunberg SM, Gralla RJ, et al. (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21:4112–4119
Takahashi T, Hoshi E, Takagi M, et al. (2011) Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci 11:2455–2461
Saito M, Aogi K, Sekine I, et al. (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Longo F, Mansueto G, Lapadula V, et al. (2011) Palonosetron plus 3-day aprepitant and dexamethasone to prevent nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 19:1159–1164
Tamura K, Aiba K, Saeki T, et al. (2015) Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol 2015 Feb. 15.
Takeshima N, Matoda M, Abe M, et al. (2014) Efficacy and safety of triple therapy with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy for gynecological cancer: KCOG-G1003 phase II trial. Support Care Cancer 22:2891–2898
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9:188–195
Tan L, Liu J, Liu X, et al. (2009) Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 28:131–137
Liu J, Tan L, Zhang H, et al. (2014) QoL evaluation of olanzapine for chemotherapy-induced nausea and vomiting comparing with 5-HT3 receptor antagonist. Eur J Cancer Care 2014 Nov. 18.
Navari RM, Nagy CK, Gray SE (2013) The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21:1655–1663
Abe M, Komeda S, Kuji S, et al. (2013) Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting resistant to standard antiemetic treatment for highly emetogenic chemotherapy. Palliat Care Res 8:127–134
Abe M, Kasamatsu Y, Kado N, et al. (2015) Efficacy of olanzapine combined therapy for patients receiving highly emetogenic chemotherapy resistant to standard antiemetic therapy. Biomed Res Int (in Press).
Italian Group for Antiemetic Research (2000) Prevention of cisplatin-induced delayed emesis: still unsatisfactory. Support Care Cancer 8:229–232
Roscoe JA, Morrow GR, Aapro MS, et al. (2011) Anticipatory nausea and vomiting. Support Care Cancer 19:1533–1538
Morrow GR, Roscoe JA, Hickok JT, et al. (1998) Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology (Williston Park) 12:32–37
Passik SD, Navari RM, Jung SH, et al. (2004) A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Investig 22:383–388
Navari RM, Einhorn LH, Passik SD, et al. (2005) A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer 13:529–534
Navari RM, Einhorn LH, Loehrer PJ Sr., et al. (2007) A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer 15:1285–1291.
Bymaster F, Perry KW, Nelson DL, et al. (1999) Olanzapine: a basic science update. Br J Psychiatry 37:36–40
Passik SD, Lundberg J, Kirsh KL, et al. (2002) A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manag 23:526–532
Kast RE, Foley KF (2007) Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care 16:351–354
Kaneishi K, Kawabata M, Morita T (2012) Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manag 44:604–607
Navari RM, Brenner MC (2010) Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 18:951–956
Pleuvry BJ (2003) Physiology and pharmacology of nausea and vomiting. Anaesth Intensive Care 4:349–352
Arakawa R, Okumura M, Ito H, et al. (2010) Positron emission tomography measurement of dopamine D2 receptor occupancy in the pituitary and cerebral cortex: relation to antipsychotic-induced hyperprolactinemia. J Clin Psychiatry 71:1131–1137
Acknowledgments
We would like to express our gratitude to Tatsuya Morita, M.D., from Seirei Mikatahara General Hospital, Satoru Iwase, M.D., from Tokyo University, and members of the KCOG for advising us regarding the creation of this trial protocol. The authors thank Crimson Interactive Pvt. Ltd. (Ulatus)—www.ulatus.jp—for their assistance in manuscript translation and editing.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abe, M., Hirashima, Y., Kasamatsu, Y. et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer 24, 675–682 (2016). https://doi.org/10.1007/s00520-015-2829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2829-z