Abstract
Purpose
The aim of this project was to develop clinical practice guidelines on the use of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the prevention and management of oral mucositis (OM) in cancer patients.
Methods
A systematic review of the available literature was conducted. The body of evidence for the use of each agent, in each setting, was assigned a level of evidence. Based on the evidence level, one of the following three guideline determinations was possible: recommendation, suggestion, or no guideline possible.
Results
A recommendation was developed in favor of patient-controlled analgesia with morphine in hematopoietic stem cell transplant (HSCT) patients. Suggestions were developed in favor of transdermal fentanyl in standard dose chemotherapy and HSCT patients and morphine mouth rinse and doxepin rinse in head and neck radiation therapy (H&N RT) patients. Recommendations were developed against the use of topical antimicrobial agents for the prevention of mucositis. These included recommendations against the use of iseganan for mucositis prevention in HSCT and H&N RT and against the use of antimicrobial lozenges (polymyxin–tobramycin–amphotericin B lozenges/paste and bacitracin–clotrimazole–gentamicin lozenges) for mucositis prevention in H&N RT. Recommendations were developed against the use of the mucosal coating agent sucralfate for the prevention or treatment of chemotherapy-induced or radiation-induced OM. No guidelines were possible for any other agent due to insufficient and/or conflicting evidence.
Conclusion
Additional well-designed research is needed on prevention and management approaches for OM.
Similar content being viewed by others
Introduction
Oral mucositis (OM) is a highly significant and potentially dose-limiting complication of cancer therapy. The morbidity of OM is primarily due to pain associated with the oral mucosal inflammation and ulceration. Mucositis pain negatively affects oral intake including dietary intake and oral medications, maintenance of oral hygiene, and quality of life. Therefore, there has been significant interest in the use of agents that can alleviate mucositis-associated pain. Patients with OM are often prescribed a mouthwash containing a topical amide anesthetic agent such as lidocaine, usually in combination with other agents. Such use of a topical anesthetic may provide short-term pain relief and can facilitate eating and oral care in some patients with mild–moderate mucositis. The limited duration of effect and side effects of use limit the utility of these combination agents. Most patients with moderate–severe OM require systemic analgesics, commonly including opioids. These potent systemic analgesics are associated with significant side effects. Therefore, some studies have evaluated various topical agents, including topical formulations of analgesics, for mucositis pain. Additionally, topical coating agents that may protect the mucositis lesions have also been studied. The general rationale for these agents is that they are hypothesized to reduce pain and facilitate healing by covering the mucosal ulcerations and exposed nerve endings. Another concern with mucositis lesions relates to colonization of the oral ulcerations by microbial flora. While mucositis is not of infectious etiology, secondary microbial colonization of oral lesions can cause clinically relevant local or systemic infection and can theoretically exacerbate mucositis severity. Therefore, antimicrobial agents have also been evaluated for their effect on OM.
While there is a growing body of literature on these agents, the results are frequently conflicting. To support evidence-based patient management and improve clinical outcomes, the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) has published evidence-based clinical practice guidelines for mucositis [48]. The purpose of this systematic review is to update the published MASCC/ISOO mucositis guidelines in relation to antimicrobial, mucosal coating, anesthetic, and analgesic agents [7].
Methods
The methods are described in detail in the papers by Bowen et al. [14] and Elad et al. [30]. Briefly, a literature search for relevant papers published before December 31, 2010 was conducted using Ovid MEDLINE, with papers selected for review based on defined inclusion and exclusion criteria. The search was designed to focus on the use of antimicrobials, coating agents, anesthetics, and analgesics for OM. The list of intervention keywords used for this section included the following: acyclovir, amitriptyline, adhesive, amphotericin B, analgesic, analgesia, antacid, antibiotic, anti-infective, alfentanil, aqua oral, benzocaine, coating agent, clarithromycin, diclosan, doxepin, fentanyl, film, fluconazole, gabapentin, IB-367, hydromorphone, iseganan, kaopectate, ketamine, kefir, lidocaine, local anesthetic, “magic” or “miracle” mouthwash, mouth rinse or mouthwash, mucoadhesive, methadone, morphine, nystatin, patient controlled, polymyxin, povidone–iodine, polyvinylpyrrolidone, protegrin, sucralfate, tetracaine, tetracycline, tobramycin, topical, zilactin, xylocaine. In addition, the brand names of commercial products in these categories were also searched, including Gelclair, MuGard, and UlcerEase.
The titles of identified papers and their abstracts were reviewed in order to select those that met the inclusion criteria. The identified papers were reviewed by two independent reviewers and data were extracted using a standard electronic form. Studies were evaluated based on the list of major and minor flaws published by Hadorn [42]. A level of evidence was assigned for each intervention based on the Somerfield criteria [75]. A well-designed study was defined as a study with no major flaws as per the Hadorn criteria. However, for studies of medications used to manage mucositis pain, the lack of a placebo group was not considered to be a major flaw as it would not be ethical to provide no pain medication. Findings from the reviewed studies were integrated into guidelines based on the overall level of evidence for each intervention. Guidelines were classified into three types: recommendation, suggestion, and no guideline possible. Guidelines were separated based on (1) the aim of the intervention (prevention or treatment of mucositis); (2) the treatment modality (radiotherapy, chemotherapy, chemoradiotherapy, or high-dose conditioning therapy for hematopoietic stem cell transplant [HSCT]); and (3) the route of administration of the intervention.
Results
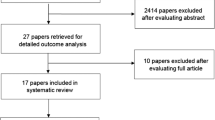
The literature searches identified 1,384 papers for which the abstracts were reviewed. Of these, 145 papers were retrieved for detailed review. Six articles were removed after the evaluation of the full article based on the inclusion/exclusion criteria described in Bowen et al. The remaining 62 papers were included in the systematic review. Table 1 presents the summary information of these publications.
Antimicrobials
Acyclovir
Acyclovir is an antiviral drug, commonly used for the treatment of herpes simplex virus (HSV) type 1 and 2 infections and in the treatment of varicella zoster (chickenpox). Only two studies were identified that studied the effects of acyclovir on the prevention of OM. A randomized controlled trial (RCT) examined the effect of acyclovir prophylaxis (or no prophylaxis) on the occurrence of oral ulcers in 74 HSV seropositive patients with acute myelogenous leukemia receiving remission induction chemotherapy. Patients receiving acyclovir prophylaxis were less likely to experience oral ulceration [12]. However, the study did not clearly differentiate between mucositis and lesions due to HSV reactivation. In contrast, another RCT reported no difference in the frequency and type of mouth lesions in 57 head and neck (H&N) cancer patients treated with chemotherapy or radiation therapy (RT), with or with acyclovir prophylaxis [15]. Due to the conflicting results and the difficulty of clinically separating viral lesions from toxicity-induced mucositis and insufficient evidence, no guideline was possible related to the use of acyclovir in preventing OM. However, the panel recognized that acyclovir is useful in preventing recurrent herpetic lesions in HSV seropositive patients undergoing highly immunosuppressive chemotherapy.
Clarithromycin
Macrolide antibiotics such as erythromycin, clarithromycin, and azithromycin have antibacterial as well as immunomodulatory activities. In an animal model, clarithromycin was found to significantly reduce the incidence of cyclophosphamide-induced intestinal mucositis [84]. An open-label prospective study examined the effect of clarithromycin prophylaxis on OM secondary to conditioning chemotherapy for bone marrow transplant (BMT). Patients receiving clarithromycin experienced severe OM less frequently than untreated controls (n = 35 in each group, p < 0.05). The authors suggested that, in addition to its antimicrobial effect, clarithromycin may improve the phagocytic function of macrophages and, thus, promote healing [86]. No guideline was possible due to insufficient evidence.
Nystatin
Polyene antifungals, such as nystatin and amphotericin B, bind to sterols in fungal cell membranes, increasing permeability and causing leakage of cell constituents. In a RCT of leukemia patients treated with chemotherapy or BMT, the use of nystatin mouth rinse, alone (n = 16) or in combination with chlorhexidine (n = 34), did not reduce the severity of OM, as compared to a saline rinse (n = 18). Fifty-six percent of subjects in the study were also given intravenous amphotericin B because of persistent fever while receiving broad-spectrum antibiotics [32]. No guideline was possible due to insufficient evidence.
Triclosan
Triclosan is a broad-spectrum antibacterial agent that increases the permeability of the bacterial cell wall. It may also have anti-inflammatory properties, suggested by in vitro studies in which triclosan blocked prostaglandin E2 production in human gingival fibroblast cultures [50]. A RCT of 24 H&N cancer patients undergoing radiotherapy compared 0.3 % triclosan rinse to sodium bicarbonate rinse, both started on the development of oral mucosal erythema. While all subjects developed ulcerative OM, stage 4 mucositis (inability to tolerate oral intake) occurred in only one subject in the triclosan group as compared to ten subjects in the control group. The triclosan group also experienced less weight loss and faster healing of grade 3 mucositis as compared to the control group [70]. No guideline was possible due to insufficient evidence.
Kefir
Kefir, which is a fermented milk complex, includes probiotic bacteria and has demonstrated in vitro antimicrobial activity against a wide variety of bacteria and some fungi. Kefir has also been reported to stimulate the immune system, based on in vitro and animal studies. A RCT of 37 colorectal cancer patients undergoing chemotherapy, with or without intestinal RT, tested an oral lavage of Kefir which was then swallowed, as compared to a saline rinse. There was no difference in OM incidence or severity and also no difference in serum levels of pro-inflammatory cytokines between the two arms [80]. No guideline was possible due to insufficient evidence.
Iseganan
Iseganan is an analog of protegrin-1, a naturally occurring beta-defensin peptide with broad-spectrum microbicidal activity. Iseganan kills a broad-spectrum of bacteria and fungi, including those resistant to conventional antimicrobial drugs, by damaging the integrity of the microbial lipid cell membrane. The first RCT examining iseganan for chemotherapy-induced OM suffered from a significant drug dispensing error due to a flawed computerized allocation system [38]. A second RCT examined the effect of rinsing with iseganan or placebo mouthwash (n = 251 in each arm) on OM in a population of mostly HSCT patients, receiving high-dose chemotherapy, with or without total body irradiation (TBI). There was no significant difference between the two arms in OM incidence or severity, peak mouth pain, peak difficulty swallowing, or amount of opioid analgesics used [39]. A separate RCT in H&N cancer patients receiving RT or chemoradiotherapy, compared iseganan (n = 253) or placebo mouth rinse (n = 171), both in combination with standard oral hygiene, against standard oral hygiene alone (n = 87). In this population as well, iseganan use did not reduce the incidence or severity of OM [81]. Each of these two large multicenter RCTs provided level II evidence, which allowed the development of a recommendation against the use of iseganan for the prevention of OM in both these populations. The panel recognized that the commercial development of iseganan has been discontinued.
Previous guideline
None.
New guideline
The panel recommends that iseganan mouthwash should not be used for the prevention of OM in HSCT patients receiving high-dose chemotherapy with or without TBI (level of evidence II) or in patients receiving H&N RT or chemoradiotherapy (level of evidence II).
Povidone–iodine
Povidone–iodine is a broad-spectrum antimicrobial agent that is typically used topically to disinfect skin wounds. An unblinded RCT of 40 H&N cancer patients undergoing chemoradiation reported that rinsing the mouth with povidone–iodine resulted in reduced incidence of OM as compared to rinsing with sterile water (n = 20 in each group) [1, 68]. Another study, in patients receiving chemoradiation for esophageal cancer, evaluated an oral hygiene regimen consisting of povidone–iodine mouth rinse in combination with toothbrushing with a special brush, irrigation, suctioning, and cleaning of oral mucosal surfaces, all performed by a dentist. It was found that the subjects receiving this special oral hygiene regimen had a significantly lower incidence of OM as compared to a control group which consisted of basic toothbrushing on their own (n = 20 in each group) [85]. On the other hand, a multicenter RCT with 132 HSCT patients found no difference between mouth rinsing with povidone–iodine or saline in OM, fever of unknown origin, or other infections [82]. No guideline was possible due to insufficient and conflicting evidence.
Combination antimicrobial lozenge or paste
A number of studies have assessed a combination of various antimicrobial agents used topically in the oral cavity for the prevention of OM. The combination of polymyxin, tobramycin, and amphotericin B (PTA) has been tested in multiple studies in H&N cancer patients receiving RT, either as a lozenge or as a paste. Polymyxins are produced by the gram-positive bacterium Bacillus polymyxa and are selectively toxic for gram-negative bacteria. Tobramycin is an aminoglycoside antibiotic used to treat various types of bacterial infections, particularly gram-negative infections. Amphotericin B is a polyene antifungal, often used intravenously for systemic fungal infections. Although initial pilot studies suggested the potential efficacy of this combination, subsequent larger well-controlled studies clearly demonstrated that topical use of PTA did not prevent OM or reduce its severity [76, 77, 79, 83]. In addition, a well-controlled, multicenter, double-blind RCT examined the effect of a lozenge containing bacitracin, clotrimazole, and gentamicin (BCoG) for the prevention of radiation-induced OM in 137 H&N cancer patients. No difference was found in the extent of severe mucositis or in the time to development of severe mucositis [31]. The previously discussed body of evidence continued to support a recommendation against the use of these combined antimicrobial preparations for the prevention of OM.
Previous guideline
The panel recommends that antimicrobial lozenges not be used for the prevention of radiation-induced OM (level II evidence).
New guideline
The panel recommends that PTA and BCoG antimicrobial lozenges and PTA paste should not be used for the prevention of radiation-induced OM in H&N cancer patients (level II evidence).
Fluconazole
Fluconazole is a triazole antifungal drug used in the prevention and treatment of superficial and systemic fungal infections. An open-label, nonrandomized study compared a cohort of 34 patients who received daily fluconazole prophylaxis during H&N RT with another cohort of 29 patients who received fluconazole for 1 week only upon the development of clinical candidiasis. At the end of radiotherapy, severe OM was found less frequently in the prophylaxis group and they also had a significantly lower rate of treatment interruptions [62]. On the other hand, Corvo et al. found that the use of fluconazole reduced the incidence of oropharyngeal candidiasis but had no impact on oral mucosal toxicity [26]. No guideline was possible due to insufficient evidence.
Mucosal coating agents
Sucralfate
Sucralfate is an aluminum salt of sulfated sucrose. It has been used since 1968 for the treatment of duodenal ulcers [9]. The mechanism of action of sucralfate as an antiulcer agent is proposed to be due to the formation of an ulcer-adherent complex with proteinaceous exudates, such as albumin, at the ulcer site. The resulting adhesive barrier can cover and protect the mucosal surface. A total of 20 studies evaluated the effects of sucralfate on mucositis. The dosage varied between studies; from 1 g/15 ml suspension to a 12-g suspension, with frequency of use ranging from t.i.d. to q.i.d. Sucralfate was used as a mouthwash in all studies and subsequently swallowed in some studies.
Prevention of radiation-induced oral mucositis
Eight studies evaluated sucralfate for the prevention of radiation-induced OM in H&N cancer patients [20, 33, 36, 37, 53, 57, 58, 69]. Although some lower-level studies suggested an effect, multiple well-designed RCTs clearly demonstrated no benefit of sucralfate on mucositis severity or pain relief. One additional study evaluated sucralfate for the prevention of OM in H&N cancer patients receiving either RT or concomitant chemoradiation. This well-designed, double-blind, RCT with 102 subjects found no difference between the sucralfate and placebo groups in mucositis severity, pain, nutritional intake, weight loss, or need for treatment breaks [18]. Collectively, this body of evidence supported a new recommendation against the use of sucralfate in this setting.
Previous guideline
None.
New guideline
The panel recommends that sucralfate mouthwash should not be used for the prevention of OM in H&N cancer patients receiving RT (level of evidence I) or concomitant chemoradiation (level of evidence II).
Treatment of radiation-induced oral mucositis
Four studies tested sucralfate for the treatment of radiation-induced OM in H&N cancer patients, with the intervention started after the onset of mucositis [9, 28, 59, 65]. Only an uncontrolled case series reported a possible benefit of sucralfate. The remaining three studies, including a well-designed RCT, found no benefit of sucralfate on mucositis severity or pain. The collective evidence continued to support a recommendation against the use of sucralfate in this setting.
Previous guideline
The panel recommends that sucralfate not be used for the treatment of radiation-induced OM (level II evidence).
New guideline
The panel recommends that sucralfate mouthwash should not be used for the treatment of OM in H&N cancer patients receiving RT (level II evidence).
Prevention of chemotherapy-induced oral mucositis
Five studies tested sucralfate for the prevention of chemotherapy-induced OM, four in standard dose chemotherapy and one in HSCT patients [19, 40, 63, 66, 73]. Only one uncontrolled study reported a beneficial effect, based on a comparison to previous literature. The remaining four studies, including two well-designed RCTs, all found no benefit of sucralfate on signs or symptoms of OM. Collectively, this body of evidence supported a new recommendation against the use of sucralfate in this setting.
Previous guideline
None.
New guideline
The panel recommends that sucralfate mouthwash should not be used for the prevention of OM in patients receiving chemotherapy (level I evidence).
Treatment of chemotherapy-induced oral mucositis
Two studies evaluated tested sucralfate for the treatment of chemotherapy-induced OM. A double-blind RCT with 131 subjects receiving 5-fluorouracil-based chemotherapy found no difference in mucositis severity or duration between the sucralfate and placebo groups [55]. A second double-blind RCT with 40 subjects receiving chemotherapy also reported no difference in pain scores or resolution of mucositis lesions [23]. These two well-designed RCT studies supported a recommendation against the use of sucralfate in this setting.
Previous guideline
None.
New guideline
The panel recommends that sucralfate mouthwash should not be used for the treatment of OM in patients receiving chemotherapy (level I evidence).
Gelclair
Gelclair is a polyvinylpyrrolidone–sodium hyaluronate gel that is marketed as a medical device for the management of mucositis pain. It is applied to the surface of the oral mucosa in the form of a viscous bioadherent gel that is proposed to form a protective layer over the mucosa. In a RCT of H&N cancer patients with radiation-induced OM, 20 subjects received either Gelclair or standard therapy consisting of sucralfate and topical marcaine over a 24-h period. No significant difference was found between the Gelclair and the standard therapy arms in mouth pain, pain on speaking, or capacity to eat or drink [8]. No guideline was possible due to insufficient evidence.
Anesthetics
Tetracaine
An uncontrolled study examined the use of an oral gel containing tetracaine in 50 subjects with radiation-induced OM. In a questionnaire, 79 % of the subjects reported a reduction in oral pain with use of the gel. The planned course of radiation was interrupted less frequently in patients who reported a benefit from gel application than in those who did not [3]. No guideline was possible due to insufficient evidence.
Amethocaine
Amethocaine hydrochloride, a local anesthetic, was assessed in a randomized controlled study enrolling 38 patients undergoing RT. The study aimed at pain relief, and only oral symptoms were assessed [54]. No guideline was possible due to insufficient evidence.
Dyclonine
A prospective double-blind study was conducted on 18 cancer patients undergoing either chemotherapy or radiotherapy. Relief of pain due to OM was assessed with one of four agents: dyclonine, lidocaine, cocaine, a combination rinse (kaolin–pectin, diphenhydramine, and saline), and a placebo solution. The results indicated that dyclonine provided the greatest degree of pain relief but was the least palatable of the tested agents [17]. No guideline was possible due to insufficient evidence.
MGI-209 (containing benzocaine)
MGI-209 is a hydroxypropyl cellulose gel that contains the topical anesthetic agent benzocaine hydrochloride (15 %). It is proposed to form a mucoadherent protective coating on the oral mucosa. In an open-label uncontrolled study of 28 subjects undergoing cytotoxic chemotherapy, the use of MGI-209 resulted in reduced pain scores for up to 180 min [52]. No guideline was possible due to insufficient evidence.
Cocaine
Cocaine was the first local anesthetic to be used clinically but carries a significant risk of dependence and abuse. A case report examined the use of a 4 % cocaine topical solution in two individuals with opioid-resistant severe pain due to mucositis from chemoradiation for oral cancer. Both individuals reported that the topical cocaine, applied directly on the oral ulcerations, produced a rapid and dramatic pain relief [61]. No guideline was possible due to insufficient evidence.
Analgesics
Capsaicin
Capsaicin is the active ingredient in chili peppers. It desensitizes some neurons by depletion of substance P and has been reported to provide moderate pain relief on the skin. In an open-label, uncontrolled study of 11 patients with pain due to cancer therapy-induced OM, the use of capsaicin lozenges resulted in a reduction in pain scores in all subjects. However, this pain relief was temporary and not complete for most patients [11]. No guideline was possible due to insufficient evidence.
Methadone
Methadone is a synthetic opioid that interacts with opioid receptors in the central nervous system and also on peripheral nerves. A case report suggested that mucositis-related pain may be effectively treated with sublingual methadone due to peripheral and/or central mechanisms [41]. No guideline was possible due to insufficient evidence.
Ketamine
Ketamine selectively blocks afferent impulses of pain perception. It may have utility in pain states associated with hyperalgesia and allodynia, including neuropathic pain, burns, and inflammatory disorders. A retrospective record review found that the addition of ketamine to a morphine infusion improved analgesic efficacy in 16 children with mucositis pain, with no increase in side effects [47]. A case report of one H&N cancer patient receiving concomitant chemoradiation reported that ketamine oral rinse was highly effective in decreasing mouth pain at rest and with eating [74]. No guideline was possible due to insufficient evidence.
Patient-controlled analgesia
The term patient-controlled analgesia (PCA) typically refers to an intravenous administration of an opioid analgesic, such as morphine or hydromorphone, where the patient has the ability to self-administer a bolus dose of the analgesic when needed to control pain. Seven RCTs have examined the use of PCA for the management of pain due to OM in patients undergoing HSCT [24, 25, 43–46, 56, 67]. Studies compared either different opioids delivered via PCA, or PCA compared to a continuous infusion only, or different modes of monitoring. All seven studies found PCA to be an effective mode of opioid administration in the management of mucositis pain. Studies comparing PCA to a continuous infusion reported that total opioid use was lower in patients receiving PCA [43, 56, 67]. One study examined the use of a PCA system where patients adjusted the rate of a continuous morphine infusion to increase or decrease their plasma morphine concentration. The group using such pharmacokinetically based PCA reported greater pain relief than patients using typical PCA [44]. A well-designed double-blind RCT compared PCA with morphine, hydromorphone, and sufentanil. Although analgesia achieved in all three groups was nearly equivalent, morphine had fewer side effects and a lower dose requirement to achieve pain control [24]. This body of evidence supported the continuation of a recommendation in favor of PCA.
Previous guideline
The panel recommends PCA with morphine as the treatment of choice for OM pain in patients undergoing HSCT (level of evidence II).
New guideline
The panel recommends PCA with morphine for the management of pain due to OM in patients undergoing HSCT (level of evidence II).
Fentanyl
Fentanyl is a synthetic opioid analgesic with strong agonist activity at the μ-opioid receptor. Fentanyl is approximately 100 times more potent in analgesic activity than morphine. Due to its rapid onset and short duration of action, fentanyl is used as a transdermal patch that delivers a steady continuous dose of medication. Four studies have evaluated transdermal fentanyl for the management of pain due to OM secondary to standard dose chemotherapy or high-dose chemotherapy prior to HSCT [16, 27, 49, 78]. One study reported a lack of efficacy but only examined the 25- and 50-μg dose levels, whereas in practice, patients can be titrated up to higher doses if needed for pain relief. The other three studies all reported that transdermal fentanyl was highly effective in producing pain relief. This body of evidence supported a new suggestion in favor of transdermal fentanyl in this setting. Transmucosal fentanyl was also studied and the tolerability and effects of two formulations assessed in patients with radiation-induced OM. No guideline was possible due to insufficient evidence and the concern of patient safety in this delivery method [71].
Previous guideline
None.
New guideline
The panel suggests that transdermal fentanyl can be effective for the management of pain due to OM secondary to standard dose chemotherapy or high-dose chemotherapy prior to HSCT (level of evidence of III).
Topical morphine
The administration of morphine sulfate extended-release capsules via gastrostomy was reviewed in a population of H&N cancer patients. While these studies suggested effective pain relief, no guideline was possible due to insufficient evidence [72]. In addition to its effects in the central nervous system, morphine can also have peripheral effects when applied on mucosal surfaces. A case series reported that the topical administration of a 0.08 % morphine gel provided pain relief in six cases of either cutaneous or oral mucosal pain [51]. Two studies, including a RCT, examined the use of a 2 % morphine mouth rinse for the management of pain due to OM in patients receiving chemoradiation for H&N cancer [21, 22]. In the RCT, morphine mouth rinse was compared to a “magic mouthwash” containing lidocaine, diphenhydramine, and magnesium aluminum hydroxide. The subjects receiving the morphine mouth rinse experienced a significantly lower intensity and duration of mouth pain, lower duration of severe functional impairment, and a lower need for systemic opioid analgesics. No adverse events were reported with the use of the morphine mouth rinse, which was not swallowed. These studies supported a new suggestion in favor of morphine mouth rinse in this setting.
Previous guideline
None.
New guideline
The panel suggests that morphine 2 % mouth rinse can be effective for the management of pain due to OM in patients receiving chemoradiation for H&N cancer (level of evidence III).
Nortriptyline
Nortriptyline is a tricyclic antidepressant that also has analgesic properties. Tricyclic antidepressants may be used in the treatment of various chronic pain states including neuropathic pain. A RCT compared the systemic administration of nortriptyline to that of morphine for the management of pain due to radiation-induced OM in 39 H&N cancer patients. The majority of subjects in the nortriptyline arm did not achieve adequate pain control with this agent alone, and morphine was added to their regimens [29]. No guideline was possible due to insufficient evidence.
Gabapentin
Gabapentin is an analog of the neurotransmitter gamma-aminobutyric acid. It is indicated for neuropathic pain and seizures. The results of the systematic review on the use of gabapentin for mucosal pain yielded two retrospective studies from the same institution that assessed gabapentin for the relief of pain secondary to radiation-induced OM in H&N cancer patients. One publication reported data from 30 patients undergoing RT and another study reported data from 42 patients receiving chemoradiation. The efficacy of gabapentin was assessed by examining the need for opioids for pain relief. The authors concluded that gabapentin can provide pain relief and reduce the need for opioids [5, 6]. No guideline was possible due to insufficient evidence.
Doxepin
Doxepin is another tricyclic antidepressant, with analgesic properties. Two uncontrolled studies have examined the use of a doxepin mouth rinse for the management of pain due to OM secondary to chemotherapy or RT [34, 35]. Both studies reported a strong beneficial effect of doxepin, with pain relief reported within 5 min of use and persisting for up to 6 h. In addition, pain control was improved following repeat dosing, suggesting a potential for a cumulative effect over time despite increasing severity of tissue damage due to mucositis over the study period. The consistent positive results of these two studies supported a new suggestion in favor of doxepin mouth rinse.
Previous guideline
None.
New guideline
The panel suggests that 0.5 % doxepin mouth rinse may be effective for the management of pain due to OM (level of evidence IV).
Discussion
As demonstrated by the results of this systematic review, a wide variety of agents have been evaluated for the prevention or treatment of OM secondary to cancer therapy. However, for most agents, the evidence was insufficient to support a guideline for or against the use of the agent. Nevertheless, we were able to formulate several new guideline statements in relation to some agents within the classes reviewed here.
A number of antimicrobial agents have been studied for OM, including antibacterial, antiviral, and antifungal agents. The best studied of these is iseganan for which large multicenter phase III studies were conducted as part of a commercial drug development program. Unfortunately, these studies convincingly demonstrated a lack of benefit from this agent, leading to the development of a recommendation against its use both in chemotherapy-induced and radiation-induced OM. Multiple studies of combination topical antimicrobial formulations (PTA and BCoG antimicrobial lozenges and PTA paste) for radiation-induced OM in H&N cancer patients also demonstrated no benefit, leading to a recommendation against the use of these agents. No guideline was possible for any of the other antimicrobial agents reviewed.
Chlorhexidine has been researched for mucositis as well. The detailed findings of the systematic review related to chlorhexidine will be presented in the paper by McGuire et al, elsewhere in this issue. However, in summary, the guidelines suggest that chlorhexidine mouthwash not be used in the prevention of OM in adult patients with H&N cancer who are undergoing radiotherapy. No guideline is possible for the use of chlorhexidine mouthwash in the prevention or treatment of OM in any other population due the insufficient and/or conflicting evidence. One cannot discount the use of chlorhexidine as an effective antiplaque agent in the role oral decontamination.
Overall, the results of studies of antimicrobial agents demonstrate that a nonclinical secondary colonization of mucositis lesions does not seem to play an important role in the pathogenesis of OM. However, in some settings, a secondary clinical infection can result in lesions that may mimic mucositis and complicate its diagnosis. An example would be recurrent HSV lesions in patients receiving myeloablative chemotherapy. In such situations, the use of antiviral prophylaxis can be warranted, not for mucositis, but to prevent or treat herpetic stomatitis. Similarly, the high prevalence of clinical oral candidiasis during H&N radiotherapy and the potential for fungal infection to worsen the severity of mucositis provide a rationale for testing the effect of antifungal prophylaxis or treatment on mucositis. It is a matter of debate if the greater benefit on mucositis could result from fungal decolonization or from treatment of overt infection. However, available studies on this approach were limited.
A number of mucosal coating agents have been commercially marketed for OM. However, there was almost no published data available for these agents. In contrast, the single most studied agent we reviewed here was sucralfate, which is a mucosal coating agent. Twenty published studies have tested sucralfate in OM in various populations. These studies clearly demonstrated a lack of benefit for sucralfate in the prevention or treatment of OM secondary to chemotherapy or RT. The evidence supported four recommendations against the use of sucralfate in these various settings. These data indicate that forming a protective coating over the oral mucosa does not prevent or treat OM. On the other hand, it appears theoretically feasible that such a protective coating can protect the exposed nerve endings and, thus, reduce pain. However, the sucralfate data do not provide support for such a beneficial effect. The different mucosal coating agents require well-designed studies to assess utility in OM.
Although the use of topical anesthetic agents is very common in patients with OM, studies of such agents in isolation are limited. These agents are often used and tested in combination rinses containing a topical anesthetic, a mucosal coating agent, and other agents, sometimes including anti-inflammatory and antimicrobial agents. This makes it difficult to determine the potential benefit of any one component. Since such combination rinses are typically used as a component of an oral care protocol, they were reviewed by the section on basic oral care and will be discussed in a separate manuscript elsewhere in this issue. With regard to the few studies of topical anesthetics alone, they all demonstrated some benefit with regards to pain relief. However, the lack of high-level evidence precluded the development of any guidelines. It seems quite logical that the use of a topical anesthetic on oral ulcerations will provide some pain relief. However, such a benefit is usually transient and most patients with severe mucositis will also need systemic analgesics. Nevertheless, clinical experience suggests that the use of topical anesthetics can be useful in some patients to provide temporary relief and allow patients to carry out activities such as eating or oral hygiene. Since the benefit of other components of commonly used combination rinses is unknown, more studies of topical anesthetics in isolation are warranted.
Systemic analgesics, including opioids, are clinically used for pain management in most patients with severe OM. As can be expected, almost every study examining the use of opioid analgesics for mucositis pain demonstrated a reduction in pain. However, these agents have significant side effects and efficacy can vary by medication, dose, and route of administration. For example, transdermal absorption of fentanyl is temperature-dependent. It is important to note that despite the use of opioids, patients with severe mucositis report significant pain. Therefore, the real question in the review of these agents was which agents and which mode of administration can provide optimal pain relief with minimal side effects. With regards to hospitalized patients undergoing HSCT, the evidence supported a recommendation in favor of PCA with morphine, administered intravenously. There was also evidence to support a suggestion in favor of transdermal fentanyl administration in conventional chemotherapy and HSCT patients, which is a strategy that can be used on outpatients as well. Due to the side effects associated with the systemic use of opioids, there has been increasing interest in the use of these agents topically in the oral cavity. Recent studies have indicated that opioid receptors are upregulated in peripheral nerves in inflammatory states. Although studies with such topical use of opioids were limited, the data did support a suggestion in favor of a 2 % morphine rinse in radiation-induced OM in H&N cancer patients. Additional well-designed studies of innovative approaches to mucositis pain control should be conducted. In addition, studies of co-analgesic adjuvant therapies are needed due to the considerable pain that continues in severe mucositis despite the use of opioids.
Certain agents that are not classified as analgesics can still have analgesic properties. For example, tricyclic antidepressants such as nortriptyline and doxepin and agents like gabapentin used in neuropathic pain have been tested for the management of OM pain. Based on the results for doxepin mouth rinse, a suggestion was made in favor of this agent. It is also relevant to note that a more recent multi-institution, randomized, double-blind, placebo-controlled, phase III trial, with a crossover phase, tested the efficacy of doxepin oral rinse versus placebo for the treatment of OM pain associated with RT for their H&N cancer (>50.0 Gy). Patients who received doxepin reported a reduction in pain with doxepin versus placebo (p = 0.0009). The majority of patients elected to continue doxepin during RT for OM pain, after the double-blind, crossover portion of the study [60]. In the future, studies like these may lead to an increase in alternate use of medications outside their predictable intent as aids in chronic or neuropathic pain and depression. These agents might be useful and should be considered in patients with relative counter-indications to opioid treatment.
It should be noted that that many of the agents reviewed here were applied topically in the oral cavity. Many patients experience mucositis that is severe enough to prevent them from drinking, swallowing, or taking oral medications. Topical drug delivery on the oral mucosa as a means to provide systemic dosing following systematic absorption might provide another therapeutic option for patients with this condition. Further studies should be lead to better delivery devices such as lollipops, transoral mucosal patches as well as define the optimal viscosity of liquid agents [71]. Mode of delivery or application of placebo agents was studied in a RCT on H&N radiation and chemotherapy and HSCT patients which determined that rinses were the most acceptable formulation by patients over both thick and thin gel formulations [10]. The efficacy of oral delivery may be affected by the agent, dose, contact time, and bioavailability. Topical delivery of medication has potential advantages, including immediate delivery to the affected tissue, rapid onset of action, and increased local drug concentration with little or no systemic exposure. Challenges of topical application include limited contact time, dilution of the agent in saliva or rapid oral clearance, and the potential for an adverse taste or texture of the agent. Different areas of the oral mucosa may have different permeabilities, and loss of the mucosal barrier leads to potential direct connective tissue contact and potential for increased systemic absorption. The oral secretions and tissue have high enzymatic activity which may affect the drug or the delivery vehicle. The relatively small size of the market for oral topical medications has limited the development of innovative topical delivery approaches to date, but increasing interest due to patient need may drive further development.
References
Adamietz IA et al (1998) Prophylaxis with povidone–iodine against induction of oral mucositis by radiochemotherapy. Support Care Cancer 6(4):373–377
Allison RR et al (1995) Symptomatic acute mucositis can be minimized or prophylaxed by the combination of sucralfate and fluconazole. Cancer Invest 13(1):16–22
Alterio D et al (2006) Tetracaine oral gel in patients treated with radiotherapy for head-and-neck cancer: final results of a phase II study. Int J Radiat Oncol Biol Phys 64(2):392–395
Anonymous (2009) The clinical effectiveness of Gelclair in the management of oral mucositis. Aust Nurs J 16(9):30–33
Bar Ad V, Weinstein G et al (2010) Gabapentin for the treatment of pain related to radiation-induced mucositis in patients with head and neck tumors treated with intensity-modulated radiation therapy. Head & Neck 32(2):173–177
Bar Ad V, Weinstein G et al (2010) Gabapentin for the treatment of pain syndrome related to radiation-induced mucositis in patients with head and neck cancer treated with concurrent chemoradiotherapy. Cancer 116(17):4206–4213
Barasch A et al (2006) Antimicrobials, mucosal coating agents, anesthetics, analgesics, and nutritional supplements for alimentary tract mucositis. Support Care Cancer 14(6):528–532
Barber C et al (2007) Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: a preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support Care Cancer 15(4):427–440
Barker G et al (1991) The effects of sucralfate suspension and diphenhydramine syrup plus kaolin–pectin on radiotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol 71(3):288–293
Bellm LA et al (2001) Assessment of various topical oral formulations by bone marrow transplant recipients. Oral Oncol 37(1):42–49
Berger A et al (1995) Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J Pain Symptom Manage 10(3):243–248
Bergmann OJ et al (1995) Acyclovir given as prophylaxis against oral ulcers in acute myeloid leukaemia: randomised, double blind, placebo controlled trial. BMJ 310(6988):1169–1172
Bondi E et al (1997) Local antimicrobial therapy of oral mucositis in paediatric patients undergoing bone marrow transplantation. Oral Oncol 33(5):322–326
Bowen J, Elad S, Hutchins R, Lalla RV, for the Mucositis Study Group of MASCC/ISOO (2012). Methodology for the MASCC/ISOO Mucositis Guidelines Update. Support Care Cancer. 21(1):303–8
Bubley GJ et al (1989) Effect of acyclovir on radiation- and chemotherapy-induced mouth lesions. Antimicrob Agents Chemother 33(6):862–865
Cai Q et al (2008) Efficacy and safety of transdermal fentanyl for treatment of oral mucositis pain caused by chemotherapy. Expert Opin Pharmacother 9(18):3137–3144
Carnel SB et al (1990) Treatment of radiation- and chemotherapy-induced stomatitis. Otolaryngol Head Neck Surg 102(4):326–330
Carter DL et al (1999) Double blind randomized trial of sucralfate vs placebo during radical radiotherapy for head and neck cancers. Head Neck 21(8):760–766
Castagna L et al (2001) Prevention of mucositis in bone marrow transplantation: a double blind randomised controlled trial of sucralfate. Ann Oncol 12(7):953–955
Cengiz M et al (1999) Sucralfate in the prevention of radiation-induced oral mucositis. J Clin Gastroenterol 28(1):40–43
Cerchietti LC et al (2002) Effect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer 95(10):2230–2236
Cerchietti LC et al (2003) Potential utility of the peripheral analgesic properties of morphine in stomatitis-related pain: a pilot study. Pain 105(1–2):265–273
Chiara S et al (2001) Sucralfate in the treatment of chemotherapy-induced stomatitis: a double-blind, placebo-controlled pilot study. Anticancer Res 21(5):3707–3710
Coda BA et al (1997) Comparative efficacy of patient-controlled administration of morphine, hydromorphone, or sufentanil for the treatment of oral mucositis pain following bone marrow transplantation. Pain 72(3):333–346
Collins JJ et al (1996) Patient-controlled analgesia for mucositis pain in children: a three-period crossover study comparing morphine and hydromorphone. J Pediatr 129(5):722–728
Corvo R et al (2008) Effects of fluconazole in the prophylaxis of oropharyngeal candidiasis in patients undergoing radiotherapy for head and neck tumour: results from a double-blind placebo-controlled trial. Eur J Cancer Care (Engl) 17(3):270–277
Demarosi F et al (2004) Transdermal fentanyl in HSCT patients: an open trial using transdermal fentanyl for the treatment of oral mucositis pain. Bone Marrow Transplant 33(12):1247–1251
Dodd MJ et al (2003) Radiation-induced mucositis: a randomized clinical trial of micronized sucralfate versus salt & soda mouthwashes. Cancer Invest 21(1):21–33
Ehrnrooth E et al (2001) Randomized trial of opioids versus tricyclic antidepressants for radiation-induced mucositis pain in head and neck cancer. Acta Oncol 40(6):745–750
Elad S, Bowen J, Zadik Y, Lalla RV, for the Mucositis Study Group of MASCC/ISOO (2012). Development of the MASCC/ISOO Mucositis Guidelines: Considerations Underlying the Process. Support Care Cancer. 21(1):309–12
El-Sayed S et al (2002) Prophylaxis of radiation-associated mucositis in conventionally treated patients with head and neck cancer: a double-blind, phase III, randomized, controlled trial evaluating the clinical efficacy of an antimicrobial lozenge using a validated mucositis scoring system. J Clin Oncol 20(19):3956–3963
Epstein JB et al (1992) Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Med Oral Pathol 73(6):682–689
Epstein JB, Wong FL (1994) The efficacy of sucralfate suspension in the prevention of oral mucositis due to radiation therapy. Int J Radiat Oncol Biol Phys 28(3):693–698
Epstein JB et al (2001) Oral topical doxepin rinse: analgesic effect in patients with oral mucosal pain due to cancer or cancer therapy. Oral Oncol 37(8):632–637
Epstein JB (2008) Doxepin rinse for management of mucositis pain in patients with cancer: one week follow-up of topical therapy. Spec Care Dentist 28(2):73–77
Etiz D et al (2000) Clinical and histopathological evaluation of sucralfate in prevention of oral mucositis induced by radiation therapy in patients with head and neck malignancies. Oral Oncol 36(1):116–120
Evensen JF et al (2001) Effects of Na-sucrose octasulfate on skin and mucosa reactions during radiotherapy of head and neck cancers—a randomized prospective study. Acta Oncol 40(6):751–755
Giles FJ et al (2003) A phase III, randomized, double-blind, placebo-controlled, multinational trial of iseganan for the prevention of oral mucositis in patients receiving stomatotoxic chemotherapy (PROMPT-CT trial). Leuk Lymphoma 44(7):1165–1172
Giles FJ et al (2004) A phase III, randomized, double-blind, placebo-controlled, study of iseganan for the reduction of stomatitis in patients receiving stomatotoxic chemotherapy. Leuk Res 28(6):559–565
Giorgi F et al (1996) Sucralfate prophylaxis of fluorouracil-induced stomatitis. Tumori 82(6):585–587
Gupta A, Duckles B, Giordano J (2010) Use of sublingual methadone for treating pain of chemotherapy-induced oral mucositis. J Opioid Manag 6(1):67–69
Hadorn DC et al (1996) Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 49(7):749–754
Hill HF et al (1990) Self-administration of morphine in bone marrow transplant patients reduces drug requirement. Pain 40(2):121–129
Hill HF et al (1991) A computer-based system for controlling plasma opioid concentration according to patient need for analgesia. Clin Pharmacokinet 20(4):319–330
Hill HF et al (1991) Patient-controlled analgesic administration. A comparison of steady-state morphine infusions with bolus doses. Cancer 67(4):873–882
Hill HF et al (1992) Patient-controlled analgesic infusions: alfentanil versus morphine. Pain 49(3):301–310
James PJ, Howard RF, Williams DG (2010) The addition of ketamine to a morphine nurse- or patient-controlled analgesia infusion (PCA/NCA) increases analgesic efficacy in children with mucositis pain. Paediatr Anaesth 20(9):805–811
Keefe DM et al (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109(5):820–831
Kim JG et al (2005) Effectiveness of transdermal fentanyl patch for treatment of acute pain due to oral mucositis in patients receiving stem cell transplantation. Transplant Proc 37(10):4488–4491
Kjaerheim V, Waaler SM (1994) Experiments with triclosan-containing mouthrinses: dose response—and an attempt to locate the receptor site(s) of triclosan in the mouth. Adv Dent Res 8(2):302–306
Krajnik M et al (1999) Potential uses of topical opioids in palliative care—report of 6 cases. Pain 80(1–2):121–125
LeVeque FG et al (1992) Clinical evaluation of MGI 209, an anesthetic, film-forming agent for relief from painful oral ulcers associated with chemotherapy. J Clin Oncol 10(12):1963–1968
Lievens Y et al (1998) Does sucralfate reduce the acute side-effects in head and neck cancer treated with radiotherapy? A double-blind randomized trial. Radiother Oncol 47(2):149–153
Logan RM (2002) Oral mucositis—current concepts and management. Ann R Australas Coll Dent Surg 16:54–57
Loprinzi CL et al (1997) Phase III controlled evaluation of sucralfate to alleviate stomatitis in patients receiving fluorouracil-based chemotherapy. J Clin Oncol 15(3):1235–1238
Mackie AM, Coda BC, Hill HF (1991) Adolescents use patient-controlled analgesia effectively for relief from prolonged oropharyngeal mucositis pain. Pain 46(3):265–269
Makkonen TA et al (1994) Sucralfate mouth washing in the prevention of radiation-induced mucositis: a placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys 30(1):177–182
Matthews RH, Ercal N (1996) Prevention of mucositis in irradiated head and neck cancer patients. J Exp Ther Oncol 1(2):135–138
Meredith R et al (1997) Sucralfate for radiation mucositis: results of a double-blind randomized trial. Int J Radiat Oncol Biol Phys 37(2):275–279
Miller R et al (2013) Doxepin rinse significantly reduces mouth pain for head and neck cancer patients who receive radiation therapy. Int J Radiat Oncol Biol Phys 85(1):21
Newport K, Coyne P (2010) Topical cocaine for relief of mucosal pain. J Pain Palliat Care Pharmacother 24(2):149–151
Nicolatou-Galitis O et al (2006) Effect of fluconazole antifungal prophylaxis on oral mucositis in head and neck cancer patients receiving radiotherapy. Support Care Cancer 14(1):44–51
Nottage M et al (2003) Sucralfate mouthwash for prevention and treatment of 5-fluorouracil-induced mucositis: a randomized, placebo-controlled trial. Support Care Cancer 11(1):41–47
Okuno SH et al (1997) A randomized trial of a nonabsorbable antibiotic lozenge given to alleviate radiation-induced mucositis. Cancer 79(11):2193–2199
Pfeiffer P et al (1990) A prospective pilot study on the effect of sucralfate mouth-swishing in reducing stomatitis during radiotherapy of the oral cavity. Acta Oncol 29(4):471–473
Pfeiffer P et al (1990) Effect of prophylactic sucralfate suspension on stomatitis induced by cancer chemotherapy. A randomized, double-blind cross-over study. Acta Oncol 29(2):171–173
Pillitteri LC, Clark RE (1998) Comparison of a patient-controlled analgesia system with continuous infusion for administration of diamorphine for mucositis. Bone Marrow Transplant 22(5):495–498
Rahn R et al (1997) Povidone–iodine to prevent mucositis in patients during antineoplastic radiochemotherapy. Dermatology 195(Suppl 2):57–61
Saarilahti K et al (2002) Comparison of granulocyte–macrophage colony-stimulating factor and sucralfate mouthwashes in the prevention of radiation-induced mucositis: a double-blind prospective randomized phase III study. Int J Radiat Oncol Biol Phys 54(2):479–485
Satheeshkumar PS et al (2010) Effectiveness of triclosan in the management of radiation-induced oral mucositis: a randomized clinical trial. J Cancer Res Ther 6(4):466–472
Shaiova L et al (2004) Tolerability and effects of two formulations of oral transmucosal fentanyl citrate (OTFC; ACTIQ) in patients with radiation-induced oral mucositis. Support Care Cancer 12(4):268–273
Shaiova L et al (2007) Administration of morphine sulfate extended-release capsules via gastrostomy: dissolution study and case reports. J Palliat Med 10(5):1063–1067
Shenep JL et al (1988) Efficacy of oral sucralfate suspension in prevention and treatment of chemotherapy-induced mucositis. J Pediatr 113(4):758–763
Slatkin NE, Rhiner M (2003) Topical ketamine in the treatment of mucositis pain. Pain Med 4(3):298–303
Somerfield MR, McCrae RR (2000) Stress and coping research. Methodological challenges, theoretical advances, and clinical applications. Am Psychol 55(6):620–625
Spijkervet FK et al (1990) Mucositis prevention by selective elimination of oral flora in irradiated head and neck cancer patients. J Oral Pathol Med 19(10):486–489
Stokman MA et al (2003) Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: a double-blind randomised clinical trial. Br J Cancer 88(7):1012–1016
Strupp C et al (2000) Transdermal fentanyl during high-dose chemotherapy and autologous stem cell support. Oncol Rep 7(3):659–661
Symonds RP et al (1996) The reduction of radiation mucositis by selective decontamination antibiotic pastilles: a placebo-controlled double-blind trial. Br J Cancer 74(2):312–317
Topuz E et al (2008) Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Invest New Drugs 26(6):567–572
Trotti A et al (2004) A multinational, randomized phase III trial of iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. Int J Radiat Oncol Biol Phys 58(3):674–681
Vokurka S et al (2005) The comparative effects of povidone–iodine and normal saline mouthwashes on oral mucositis in patients after high-dose chemotherapy and APBSCT—results of a randomized multicentre study. Support Care Cancer 13(7):554–558
Wijers OB et al (2001) Mucositis reduction by selective elimination of oral flora in irradiated cancers of the head and neck: a placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys 50(2):343–352
Woo PC et al (2000) Clarithromycin attenuates cyclophosphamide-induced mucositis in mice. Pharmacol Res 41(5):527–532
Yoneda S et al (2007) Effects of oral care on development of oral mucositis and microorganisms in patients with esophageal cancer. Jpn J Infect Dis 60(1):23–28
Yuen KY et al (2001) Effects of clarithromycin on oral mucositis in bone marrow transplant recipients. Haematologica 86(5):554–555
Disclosures
The Mucositis Guidelines Update was sponsored by Helsinn Healthcare, S.A., Switzerland and BioAlliance Pharma, France. Per MASCC/ISOO policy, no industry representatives had any role in the development of the guidelines. The authors have full control of all primary data and agree to allow the journal to review these data if requested.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Saunders, D.P., Epstein, J.B., Elad, S. et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support Care Cancer 21, 3191–3207 (2013). https://doi.org/10.1007/s00520-013-1871-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1871-y