Abstract
Introduction
This purpose of this systematic review was to evaluate the literature and update our current understanding of the impact of present cancer therapies on the dental apparatus (teeth and periodontium) since the 1989 NIH Development Consensus Conference on the Oral Complications of Cancer Therapies.
Review method
A systematic literature search was conducted with assistance from a research librarian in the databases MEDLINE/PubMed and EMBASE for articles published between 1 January 1990 and 31 December 2008. Each study was independently assessed by two reviewers. Taking into account predetermined quality measures, a weighted prevalence was calculated for the prevalence of dental caries, severe gingival disease, and dental infection. Data on DMFT/dmft, DMFS/dmfs, plaque, and gingival indexes were also gathered. The level of evidence, recommendation, and guideline (if possible) were given for published preventive and management strategies.
Results
Sixty-four published papers between 1990 and 2008 were reviewed. The weighted overall prevalence of dental caries was 28.1%. The overall DMFT for patients who were post-antineoplastic therapy was 9.19 (SD, 7.98; n = 457). The overall plaque index for patients who were post-antineoplastic therapy was 1.38 (SD, 0.25; n = 189). The GI for patients who were post-chemotherapy was 1.02 (SD, 0.15; n = 162). The weighted prevalence of dental infections/abscess during chemotherapy was reported in three studies and was 5.8%.
Conclusions
Patients who were post-radiotherapy had the highest DMFT. The use of fluoride products and chlorhexidine rinses are beneficial in patients who are post-radiotherapy. There continues to be lack of clinical studies on the extent and severity of dental disease that are associated with infectious complications during cancer therapy.
Similar content being viewed by others
Introduction
Surgical resection, radio-, and chemotherapy, either used singly or in combination, are the three most common modalities used in head and neck cancer treatment. Although these modalities are effective in eradicating the tumor, they also negatively impact the normal head and neck structures surrounding the tumor. Direct damage to the oral structures (soft and hard tissue) frequently occurs from radio- and chemotherapy, and indirect damage may also arise from systemic toxicity. These oral complications may occur during and following cancer therapy and are generally grouped into two broad categories: acute and chronic. This review will focus on the acute and chronic effects of cancer therapy on the dental apparatus (i.e., teeth and periodontium). Because of the known long-term impact of cancer therapy on the dental apparatus, in particular, the increased risk of dental caries, this article will also examine the evidence for various preventive and treatment approaches of dental disease in such patients.
Another important issue covered by this review was the evidence for pre-cancer therapy dental clearance. The rationale for dental examination and treatment prior to cancer therapy is based on reports in the literature linking increased incidence of intra-therapy complications and viridans streptococcal bacteremia in patients with poor dental health [1–3]. One of the concerns is the occurrence of acute dental infections while patients are undergoing cancer therapy. Even though this has not been extensively reported in patients who are immunosuppressed from chemotherapy, from theoretical reasoning, it is possible for a minor odontogenic infection to develop into a systemic infection and result in a life-threatening event. Another frequently cited reason for pre-cancer therapy dental clearance is the risk of post-radiation jaw osteoradionecrosis in patients who receive radiation doses above 6,000 cGy in the head and neck region. The estimated incidence of osteoradionecrosis in radiated (conventional radiation) jaw bone is approximately 7% and occurs more frequently in the mandible than in the maxilla [4]. The process may occur spontaneously, may be caused by trauma (e.g., accidental injury to oral mucosa from masticatory activity or iatrogenically from dental extractions) or oral infections (e.g., periapical and periodontal infections). Due to the concern of oral and systemic sequelae from dental infection, the dentist is often part of the pre-cancer therapy work up in many treatment centers.
The purpose of this systematic review was to evaluate the literature and update our current understanding of the impact of present cancer therapies on the dental apparatus (teeth and periodontium) and the role of pre-cancer treatment dental protocols since the 1989 NIH Development Consensus Conference on the Oral Complications of Cancer Therapies [5].
Historical perspective (prior to 1990)
The historical perspective was summarized from the 1989 National Institute of Health (NIH) Development Consensus Conference on the oral complications of cancer therapies.
Dental disease and the necessity of pre-cancer therapy dental clearance
Patients with chronic dental disease and poor oral hygiene were thought to be at increased risk for the development of acute odontogenic infections and potentially life-threatening systemic infections during periods of immunosuppression, though this appeared to occur less frequently than other acute mucosal oral infections [6, 7]. Oncology centers employed empiric guidelines to prevent these odontogenic infections, which often entailed the implementation of pre-cancer treatment dental protocols. These protocols typically included restoration of carious teeth, root canal therapy (if time permits), extraction of hopeless teeth, and dental prophylaxis with or without scaling and root planning. The guidelines for endodontic care and extractions proposed during the consensus in 1990 are illustrated in Tables 1 and 2, respectively.
These guidelines varied greatly amongst centers due to the lack of outcome-oriented trials to assess efficacy of a specific pre-cancer therapy dental protocol. Ultimately, the decision on the type of dental treatment rendered was based on the clinician's assessment of the clinical and radiographic condition of the pulpal and periodontal status of the tooth involved, the time available prior to cancer treatment initiation, and patient's immune status at the time of dental treatment.
Experts concluded that larger scale prospective studies were needed to examine the risk-benefit ratio of dental procedures prior to myelosuppressive chemotherapy. In addition, studies should also focus on the extent of periodontal, pericoronal, and dental disease that were likely to cause serious complications during chemotherapy.
Long-term effect of cancer therapies on teeth and periodontium
Prior to 1990, data on the caries profile in patients who have undergone chemotherapy were limited and conflicting [8]. Some studies reported increase caries incidence, while others did not find any changes in caries activity. Similar findings were found regarding the periodontium of these patients. In patients who had received head and neck radiotherapy, the ensuing xerostomia caused by damage to the salivary glands predisposed these patients to post-radiation caries. Fluoride therapy was considered to be the best option for prevention of post-radiation caries. The role of long-term use of chlorhexidine rinses and saliva substitutes was uncertain.
Aims
The aim of the present review was to expand on the 1989 NIH Development Consensus Conference on the Oral Complications of Cancer Therapies [5]. The specific goals of this systematic review of dental disease as an oral complication of cancer therapies were as follows:
-
1.
Determine the prevalence of dental caries in cancer survivors.
-
2.
Determine prevalence of periodontal disease in cancer survivors.
-
3.
Determine the prevalence of local and systemic infections caused by dental disease during cancer therapy.
-
4.
Determine the efficacy of preventive and treatment approaches in managing dental disease in cancer survivors.
-
5.
Determine the efficacy of pre-cancer therapy dental clearance in preventing oral complications during and after cancer therapy.
Systematic review methodology
Search strategy and criteria for selecting studies
A systematic literature search was conducted with assistance from a research librarian in the databases MEDLINE/PubMed and EMBASE for articles published between 1 January 1990 and 31 December 2008. The primary outcome was to retrieve all literature containing original data on dental caries and periodontal disease and pre-cancer dental clearance protocols in cancer patients undergoing head and neck radiotherapy, chemotherapy, or combined treatment modalities.
The following publication types were eliminated from this systematic review: systematic and non-systematic reviews; microbiology studies; growth and development studies; organ transplant studies; studies eliciting dental complications through questionnaires, studies reporting data from previous publications; phase I and II studies, opinion papers, and case reports; articles published before 1990; and publications from the 1990 NCI Monographs [9] based on the 1989 NIH Development Consensus Conference on the Oral Complications of Cancer Therapies [5]. The search was limited to the English language. Gender and age were not limited.
Review methodology
Each article was independently evaluated by two reviewers (BH, CH, JJN, MS, and VM) with pilot-tested collection forms customized for reviewing dental disease data. Dental caries was assessed by the presence (Y/N), DMFT/dmft (decayed, missing, and filled teeth: DMFT for permanent, adult teeth and dmft signifying deciduous teeth), and DMFS/dmfs indexes (decayed, missing, and filled surfaces: DMFS for permanent, adult teeth and dmfs signifying deciduous teeth) if available. Periodontal health was assessed using the plaque and gingival indexes. Further data collected for each article such as type of study, blinding, presence of control group, scale validity, and sample size were used to determine quality outcomes utilized to determine the weighted prevalence of caries and dental infection. Further details of this methodology can be reviewed in the publication by Brennan MT et al. [10].
The following assumptions were made regarding cancer diagnosis and treatment modality in this review:
-
1.
There were several articles that only described the treatment modality but did not include the cancer diagnosis. If the treatment modality involved head and neck radiation, the assumption was made that this treatment was for head and neck cancer. No assumptions were made regarding the type of head and neck malignancy.
-
2.
Although, the modality of cancer treatment (i.e., radiotherapy and chemotherapy) was described in detail in the majority of the studies, many did not specify or clarify whether this was the only treatment approach. Therefore, if only one mode of antineoplastic therapy (e.g., chemotherapy, radiotherapy, and surgery) was described in the manuscript, we made the assumption that this particular treatment was the only therapy rendered, unless otherwise stated by the authors.
Results
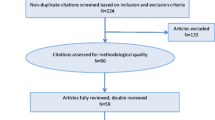
The electronic searches identified over a thousand titles and abstracts. After examination of the abstracts and full-text articles by the review group, 64 articles satisfied the inclusion criteria. Forty-six studies were observational, and 18 were interventional studies. Of the 64 studies included, 31 studies recruited adult patients, 24 recruited pediatric patients, 4 included both pediatric and adult patients, and 5 did not provide the age of the population sampled. The two most common malignancies were head and neck cancer and hematological malignancies (Table 3). The majority of studies reported the type of cancer treatment rendered (Table 4).
Observational studies
Of the 46 observational studies, 24 were cohort, 8 were case control, and 14 were cross-sectional studies. The majority of studies were conducted in single institution settings. Only one observational study did not report the type of antineoplastic therapy rendered.
-
(A)
Teeth
-
1.
Dental infection/abscess
Dental infections/abscess during chemotherapy was reported in three studies, and the weighted prevalence was 5.8% [11–13] (standard of error, 0.009; 95% confidence interval, 1.8–9.7).
-
1.
Dental caries (Table 5)
Table 5 Weighted prevalence of dental caries following cancer therapy (n = 19)
The weighted overall prevalence of dental caries was 28.1% and was determined from 19 studies [14–32]. The weighted prevalence of dental caries in patients who received only chemotherapy was 37.3%. The weighted prevalence of dental caries in patients who were post-radiotherapy and those who were post-chemo- and radiotherapy were 24% and 21.4%, respectively.
-
2.
DMFT
The overall DMFT for patients who were post-antineoplastic therapy was 9.19 (SD, 7.98; n = 457) [14, 17, 25, 29, 30, 33–36]. The DMFT for patients who were post-chemotherapy [17, 25, 33] and post-radiotherapy [14, 30, 36] was 4.5 (SD, 2.88; n = 132) and 17.01 (SD, 9.14; n = 157), respectively. The mean DMFT value in patients prior to treatment was 8.2 (SD, 0.71; n = 80) [14, 35]. The mean DMFT for healthy controls was 4.4 (SD, 4.07; N = 275) [14, 17, 25, 29, 34].
-
3.
dmft
The overall dmft for patients who were post-cancer therapy (all modalities) was 3.23 (SD, 2.42; n = 128) [29, 33, 34]. The mean dmft for healthy controls was 2.15 (SD, 1.77; n = 103) [29, 34].
-
4.
DMFT/dmft
The overall DMFT/dmft for patients who were post-chemotherapy was 5.63 (SD, 0.88; n = 66) [37, 38]. The mean DMFT/dmft for healthy controls was 4.7 (SD, 0.14; n = 56) [37, 38].
-
5.
DMFS
The overall DMFS for patients who were post-cancer therapy (all modalities) was 11.8 (SD, 9.01; n = 128) [17, 34, 35]. The mean DMFS for healthy controls was 4.1 (SD, 0.28; n = 86) [17, 34].
-
6.
DMFS/dmfs
The overall DMFS/dmfs for patients who were post-chemotherapy was 8.01 (SD, 2.14; n = 66) [37, 38]. The mean DMFS/dmfs for healthy controls was 1.08 (SD, 0.04; n = 56) [37, 38].
-
(B)
Periodontium
-
1.
Severe gingivitis
The weighted prevalence of severe gingivitis from three studies was 20.3% (standard of error, 0.49; 95% confidence interval, 0–41.4) [12, 13, 39]. All three studies were conducted on patients undergoing chemotherapy.
-
2.
Plaque index (PI)
The overall PI for patients who were post-antineoplastic therapy was 1.38 (SD, 0.25; n = 189) [25, 37, 38, 40]. The PI for patients who were post-chemotherapy was 1.46 (SD, 0.23; n = 162) [25, 37, 38]. The mean PI for healthy controls was 0.91 (SD, 0.12; n = 152) [25, 37, 38].
-
3.
Gingival index (GI)
The GI for patients who were post-chemotherapy was 1.02 (SD, 0.15; n = 162) [25, 37, 38]. The mean GI for healthy controls was 0.76 (SD, 0.10; n = 152) [25, 37, 38].
-
(C)
Febrile episodes from oral source
One study reported the incidence of patients with febrile episode originating from a dental problem was 4% [41]. Another study found that an oral source was the only identifiable foci of infection in 42% of recorded febrile episodes [42]. The same study also reported that patients with febrile episodes had more severe dental infection (57.6%) than those without (23.3%) [42].
Interventional studies
Fluoride therapy
The anticariogenic benefits of fluoride therapy are well documented in the literature. Water fluoridation has been touted to be responsible for the dramatic decrease in dental caries in the twentieth century. There are several studies that have extrapolated and investigated the benefits of fluoride use in the general population for management of dental caries in patients have undergone head and neck radiation. Because of the damage to the salivary glands during radiation and the ensuing xerostomia that follows, these patients are at higher risk that the average person for the development of dental caries. We retrieved five randomized control trials on fluoride therapy in patients who have undergone antineoplastic therapy [43–47]; four studies were on patients who were post-radiation, and one was on patients who were undergoing chemotherapy (Table 6). Two studies compared different types and methods of fluoride application on the caries activity [43, 44]. In both studies, they found no difference in caries activity between the treatment groups. Al Joburi et al. noted that patients who were noncompliant with their fluoride therapy had a significantly higher caries increment compared to the fluoride treatment groups [43]. Two articles investigated the use of an intraoral fluoride releasing system, and in both studies, there were no significant differences in dental caries between groups that used the system and those that used regular fluoride gels in custom fabricated trays [45, 46]. Meurman et al. compared the use of chlorhexidine 0.12% to amine-stannous fluoride mouth rinses in patients undergoing chemotherapy [47]. They found that both rinses reduced the gingival bleeding scores and plaque scores as well as the levels of streptococcus mutans in the saliva.
Toothpaste
There were three studies that investigated the use of various toothpastes [48–50] (Table 7). Two studies examined the benefits of toothpaste containing lactoperoxidase in patients who have undergone radiotherapy for head and neck cancer [48, 49]. Toljanic et al. found that the toothpaste containing salivary lactoperoxidase provided slight improvement in plaque and gingival index scores compared to placebo toothpaste, but this was not significant [49]. In the other study by Van Steenberghe et al., authors found that patients using toothpaste containing lactoperoxidase (Biotene) significantly reduced sulcular bleeding index after 10 days of use [48]. In addition, when only interdental spaces were considered, there was significantly lower plaque seen in the Biotene group compared to patients who were using Sensodyne toothpaste. The third article retrieved in this category compared a dual phase remineralizing toothpaste to conventional toothpaste [50]. The authors found significantly (p = 0.03) lower net root surface caries increment/year in patients using Enamelon toothpaste compared to those using conventional toothpaste.
Chlorhexidine
Chlorhexidine is a bisguanide with bactericidal activity against gram-positive and -negative bacteria. Its mechanism of action is through disruption of bacterial membranes and enzyme systems. There were three studies that evaluated the effect of chlorhexidine mouth rinse on oral hygiene indexes in patients who have undergone antineoplastic therapy [39, 47, 51] (Table 8). Two studies found that chlorhexidine rinse reduced plaque scores as well as the levels of salivary streptococcus mutans count [47, 51]. However, in both studies, the lactobacillus counts was either higher or did not change with the use of chlorhexidine. One study compared the use of chlorhexidine with and without mechanical removal of plaque and calculus on day 1 of chemotherapy and found that the plaque scores and bleeding scores were significantly lower in the group that had mechanical removal of plaque and calculus [39].
Dental restorations
There were three studies that investigated the use of various dental restorative materials in patients who have undergone head and neck radiation [52–54] (Table 9). In studies by McComb et al. [52] and Wood et al. [53], conventional glass ionomer cements performed more poorly than the comparative materials, specifically amalgam, resin-modified glass ionomer, and composite resin restorations. Hu et al. compared Ketac molar (KM) to Fuji IX (FIX) restorations and found that there was statistically (p = 0.01) higher number of KM restorations (30%) lost compared to FIX (12.5%) restorations at the 12- and 24-month follow up [54].
Others
There were two articles on oral care protocols, one [41] examined the benefits of a minimal intervention pre-cancer therapy dental protocol, and the other [55] examined the impact of an intensive preventive protocol on patients undergoing chemotherapy (Table 10). Both studies had several flaws in them, including small sample size or lack of comparison groups. There was one article each on the benefits of amifostine [56], cheese [57], and honey on dental health [58]. Results from these studies should be interpreted with caution because of the lack of additional randomized control trials.
Summary and recommendations
-
1.
The use of fluoride products reduces caries activity in patients who are post-radiotherapy. However, the type of fluoride gel or fluoride delivery system used did not significantly influence caries activity (Level of Evidence: II, Grade of Recommendation: B, Recommend the use of fluoride to prevent dental caries in patients who are post-radiotherapy).
-
2.
The use of chlorhexidine rinse reduces plaque scores and oral streptococcus mutans scores. This reduction was not seen with lactobacillus counts (Level of Evidence: II, Grade of Recommendation: B, Recommend the use of chlorhexidine to improve oral hygiene, although potential side effect of tooth staining, increased calculus, and taste changes need to be taken into account)
-
3.
There is evidence suggesting that conventional glass ionomer restorations performed more poorly that resin-modified glass ionomer, composite resin, and amalgam restorations in patients who had been treated with radiotherapy (Level of Evidence: III, Grade of Recommendation: B, Suggest the use of resin-modified glass ionomer, composite resin or amalgam restoration, and not a conventional glass ionomer restoration in patients who have been treated with radiotherapy).
-
4.
More studies are needed to determine the benefits of various types of toothpaste, pre-cancer therapy dental intervention, honey, and cheese on dental health (Level of Evidence: III, Grade of recommendation: C, No guideline possible can be made at this juncture due to the lack of well designed studies).
Discussion
In this systematic review, the weighted prevalence of dental caries amongst cancer survivors were surprisingly highest in patients who only received chemotherapy compared to those who received radiotherapy or chemoradiotherapy. This discrepancy may be attributed to the distinct differences in the dental management of patients prior to radiotherapy versus those being prepared for chemotherapy. Patients undergoing head and neck radiotherapy are at life-long risk of developing osteoradionecrosis; subsequently, dental management protocols prior to radiation often entail aggressive approaches such as extractions. Another explanation for the unanticipated caries prevalence may be because the majority of the studies were carried out on children (12/19 studies) [14–17, 20, 23–25, 27–29, 32], and a high proportion of the diagnoses in children was hematologic malignancies that were treated largely with curative chemotherapy. These children are ill for a long period of time and could have higher caries activity because of the need to frequently consume highly cariogenic dietary supplements for weight maintenance or are taking sucrose-rich medications. In addition, caregivers are often overwhelmed by their child's medical diagnosis and often neglect the oral health component. In contrast to the caries prevalence, the DMFT index is expectedly highest in patients who were post-radiation therapy compared to patients who were post-chemotherapy and healthy controls. The DMFT/S index is a means to obtain an estimation of dental disease in a population and is recommended by the World Health Organization (WHO) for the measurement of caries experience, thereby allowing for easy comparison among international studies [59]. Despite the shortcomings of the DMFT/S index (e.g., failure to detect dental decay between posterior teeth surfaces due to the lack of dental radiographs, failure to distinguish the various reasons for missing teeth) and the suggestions by several authors to switch to alternative indices, the DMFT/S index is still the most widely utilized caries assessment tool presently [60, 61]. It would have been helpful to look at the caries activity trends longitudinally in this systematic review; however, it was not possible to compile this information due to the lack of specification, standardization, and/or wide ranges of time periods of DMFT data collection.
Similarly, attempts to describe periodontal health and periodontal disease beyond that of plaque and gingival indexes in cancer patients were difficult in this review. PI is a measure of oral hygiene that synthesizes both number of surfaces covered and the amount of hard and soft deposits on the teeth, and gingival index is a measurement of the amount of inflammation present in the gingival tissues. Although, there were other measurements of periodontal health such as oral health index-simplified (OHI-S), probing depth, clinical attachment loss, gingival recession, and bleeding index, each of these parameters were only reported in a single study and therefore could not be combined or compared with other studies to have any meaningful results. Other difficulties encountered include the various reports of outcome variables (raw data versus percentages) and the categorization of periodontal health without clear definition. The measurements of DMFT/S, PI, and GI are important clinical considerations for dental practitioners because they are predictive indicators for the determination of future disease [62].
The majority of the intervention studies were carried out on patients who were post-head and neck radiotherapy, likely because these individuals are thought to be at a much higher risk for the development of dental caries compared to their post-chemotherapy counterparts. Expectedly, the use of fluoride products and chlorhexidine rinses are beneficial in reducing caries activity and levels of streptococcus mutans, respectively.
There continues to be a lack of clinical trials to evaluate the extent of dental disease associated with complications during cancer therapy, despite recommendations from the 1989 NIH consensus for more studies in this area [5]. In this review, the weighted prevalence of an odontogenic infection during chemotherapy is approximately 6%. However, these studies had small sample sizes, did not report pre-existing oral conditions, and had varied styles of reporting results, making it tricky to draw conclusions. In addition, the pre-existing oral conditions in these patients were unknown. Despite the low prevalence of dental infections, there is some evidence in the literature that these infections may cause bloodstream bacteremia and become potentially life-threatening in immunosuppressed individuals. Based on this theoretical reasoning and indirect evidence, it appears reasonable to propose that all acute and potential sources of oral infections should be eradicated. Although, large prospective studies are required to definitively address this theoretical concern for oral infection.
Another area with poor evidence is the necessity for pre-cancer therapy dental clearance, and if required, the extent of disease that needs to be eradicated. However, conducting a prospective randomized controlled trial to evaluate eradicating all oral infections prior to patients undergoing cancer therapy versus no dental treatment may likely pose ethical concerns, especially if there is sufficient time for dental clearance. Eradicating acute dental problems versus eradicating both acute and chronic dental issues may be a more practical research design. At the time of this review, there was one cohort study that examined the viability of a minimal dental intervention clearance protocol in patients prior to chemotherapy. They found a 4% conversion rate of previously diagnosed chronic dental disease to acute inter-therapy pathology and a relative incidence of 10% conversion rate of acute conversion of previously diagnosed severe chronic periodontal disease [41]. Based on their findings, the authors felt that patients with chronic dental pathology could proceed safely with chemotherapy, as the conversion rate to an acute condition was infrequent. Due to the distinct differences in the implications of the presence of dental disease in patients who are pre-chemotherapy versus those who are pre-radiotherapy, the results of this study cannot be extrapolated to patients undergoing radiotherapy. There are presently no studies that have investigated or assessed which dental treatment protocol may be the most superior and appropriate in patients undergoing radiotherapy.
Conclusions
-
1.
Patients who were post-radiotherapy had the highest DMFT compared to those who were post-chemotherapy and healthy controls.
-
2.
Patients who were post-antineoplastic therapy had higher PI and GI than healthy patients.
-
3.
The use of fluoride products and chlorhexidine rinses are beneficial in patients who are post-radiotherapy.
-
4.
Conventional glass ionomer restorations performed more poorly than resin-modified glass ionomer, composite resin, and amalgam restorations in patients who were post-radiotherapy.
-
5.
There continues to be lack of clinical studies on the extent and severity of dental disease that is associated with infectious complications during cancer therapy.
References
Graber CJ, de Almeida KN, Atkinson JC, Javaheri D, Fukuda CD, Gill VJ, Barrett AJ, Bennett JE (2001) Dental health and viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 27:537–542
Kennedy HF, Morrison D, Tomlinson D, Gibson BE, Bagg J, Gemmell CG (2003) Gingivitis and toothbrushes: potential roles in viridans streptococcal bacteraemia. J Infect 46:67–70
Peterson DE, Overholser CD (1981) Increased morbidity associated with oral infection in patients with acute nonlymphocytic leukemia. Oral Surg Oral Med Oral Pathol 51:390–393
Peterson DE, Elting LS, Spijkervet FK, Brennan MT (2010) Osteoradionecrosis in cancer patients: the evidence base for current management and new research. Oral Care Study Group, MASCC/ISOO. Support Care Cancer (in press)
National Institutes of Health (1989) Oral complications of cancer therapies: diagnosis, prevention, and treatment. National Institutes of Health Consensus Development Conference Statement, 17-19 April 1989, Vol. 7, pp 1–11
Greenberg MS (1990) Prechemotherapy dental treatment to prevent bacteremia. NCI Monogr 9:49–50
Peterson DE (1990) Pretreatment strategies for infection prevention in chemotherapy patients. NCI Monogr 9:61–71
Rosenberg SW (1990) Oral complications of cancer therapies. Chronic dental complications. NCI Monogr 9:173–178
Working Party of the British Society for Antimicrobial Chemotherapy (1982) The antibiotic prophylaxis of infective endocarditis. Lancet 1:1323–1326
Brennan MT, Spijkervet FK, Elting LS (2010) Systematic reviews of oral complications from cancer therapies, Oral Care Study Group, MASCC/ISOO: methodology and quality of the literature. Support Care Cancer. Mar 20 (in press)
Ardizzoni A, Pennucci MC, Danova M, Viscoli C, Mariani GL, Giorgi G, Venturini M, Mereu C, Scolaro T, Rosso R (1996) Phase I study of simultaneous dose escalation and schedule acceleration of cyclophosphamide-doxorubicin-etoposide using granulocyte colony-stimulating factor with or without antimicrobial prophylaxis in patients with small-cell lung cancer. Br J Cancer 74:1141–1147
Fayle SA, Curzon MEJ (1991) Oral complications in pediatric oncology patients. Pediatr Dent 13:289–295
Baliga AM, Brave VR, Vyas HA (1995) Oral mucosal lesions in patients with acute leukemias and related disorders due to cytotoxic therapy. J Indian Soc Pedod Prev Dent 13:25–29
Pow EH, McMillan AS, Leung WK, Kwong DL, Wong MC (2003) Oral health condition in southern Chinese after radiotherapy for nasopharyngeal carcinoma: extent and nature of the problem. Oral Dis 9:196–202
Estilo CL, Huryn JM, Kraus DH, Sklar CA, Wexler LH, Wolden SL, Zlotolow IM (2003) Effects of therapy on dentofacial development in long-term survivors of head and neck rhabdomyosarcoma: the memorial sloan-kettering cancer center experience. J Pediatr Hematol Oncol 25:215–222
Kaste SC, Hopkins KP, Bowman LC, Santana VM (1998) Dental abnormalities in children treated for neuroblastoma. Med Pediatr Oncol 30:22–27
Pajari U, Ollila P, Lanning M (1995) Incidence of dental caries in children with acute lymphoblastic leukemia is related to the therapy used. ASDC J Dent Child 62:349–352
Hainsworth JD, Meluch AA, McClurkan S, Gray JR, Stroup SL, Burris HA III, Yardley DA, Bradof JE, Yost K, Ellis JK, Greco FA (2002) Induction paclitaxel, carboplatin, and infusional 5-FU followed by concurrent radiation therapy and weekly paclitaxel/carboplatin in the treatment of locally advanced head and neck cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Cancer J 8:311–321
O'Sullivan EA, Duggal MS, Bailey CC (1994) Changes in the oral health of children during treatment for acute lymphoblastic leukaemia. Int J Paediatr Dent 4:31–34
Paulino AC, Simon JH, Zhen W, Wen BC (2000) Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys 48:1489–1495
Maxymiw WG, Rothney LM, Sutcliffe SB (1994) Reduction in the incidence of postradiation dental complications in cancer patients by continuous quality improvement techniques. Can J Oncol 4:233–237
Ampil FL, Nathan CO, Caldito G, Lian TF, Aarstad RF, Krishnamsetty RM (2004) Total laryngectomy and postoperative radiotherapy for T4 laryngeal cancer: a 14-year review. Am J Otolaryngol 25:88–93
Marec-Berard P, Azzi D, Chaux-Bodard AG, Lagrange H, Gourmet R, Bergeron C (2005) Long-term effects of chemotherapy on dental status in children treated for nephroblastoma. Pediatr Hematol Oncol 22:581–588
Wogelius P, Dahllof G, Gorst-Rasmussen A, Sorensen HT, Rosthoj S, Poulsen S (2008) A population-based observational study of dental caries among survivors of childhood cancer. Pediatr Blood Cancer 50:1221–1226
Avsar A, Elli M, Darka O, Pinarli G (2007) Long-term effects of chemotherapy on caries formation, dental development, and salivary factors in childhood cancer survivors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:781–789
Jham BC, Reis PM, Miranda EL, Lopes RC, Carvalho AL, Scheper MA, Freire AR (2008) Oral health status of 207 head and neck cancer patients before, during and after radiotherapy. Clin Oral Investig 12:19–24
Louis CU, Paulino AC, Gottschalk S, Bertuch AA, Chintagumpala M, Heslop HE, Russell HV (2007) A single institution experience with pediatric nasopharyngeal carcinoma: high incidence of toxicity associated with platinum-based chemotherapy plus IMRT. J Pediatr Hematol Oncol 29:500–505
Kupeli S, Varan A, Ozyar E, Atahan IL, Yalcin B, Kutluk T, Akyuz C, Buyukpamukcu M (2006) Treatment results of 84 patients with nasopharyngeal carcinoma in childhood. Pediatr Blood Cancer 46:454–458
Cubukcu CE, Sevinir B (2008) Dental health indices of long-term childhood cancer survivors who had oral supervision during treatment: a case-control study. Pediatr Hematol Oncol 25:638–646
Schwarz E, Chiu GK, Leung WK (1999) Oral health status of southern Chinese following head and neck irradiation therapy for nasopharyngeal carcinoma. J Dent 27:21–28
Walter MA, Turtschi CP, Schindler C, Minnig P, Muller-Brand J, Muller B (2007) The dental safety profile of high-dose radioiodine therapy for thyroid cancer: long-term results of a longitudinal cohort study. J Nucl Med 48:1620–1625
Raney RB, Asmar L, Vassilopoulou-Sellin R, Klein MJ, Donaldson SS, Green J, Heyn R, Wharam M, Glicksman AS, Gehan EA, Anderson J, Maurer HM (1999) Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and -III. IRS Group of the Children's Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol 33:362–371
Sonis AL, Waber DP, Sallan S, Tarbell NJ (1995) The oral health of long-term survivors of acute lymphoblastic leukaemia: a comparison of three treatment modalities. Eur J Cancer B Oral Oncol 31B:250–252
Nunn JH, Welbury RR, Gordon PH, Kernahan J, Craft AW (1991) Dental caries and dental anomalies in children treated by chemotherapy for malignant disease: a study in the north of England. Int J Paediatr Dent 1:131–135
Keene HJ, Fleming TJ, Toth BB (1994) Cariogenic microflora in patients with Hodgkin's disease before and after mantle field radiotherapy. Oral Surg Oral Med Oral Pathol 78:577–582
Duke RL, Campbell BH, Indresano AT, Eaton DJ, Marbella AM, Myers KB, Layde PM (2005) Dental status and quality of life in long-term head and neck cancer survivors. Laryngoscope 115:678–683
Oguz A, Cetiner S, Karadeniz C, Alpaslan G, Alpaslan C, Pinarli G (2004) Long-term effects of chemotherapy on orodental structures in children with non-Hodgkin's lymphoma. Eur J Oral Sci 112:8–11
Alpaslan G, Alpaslan C, Gogen H, Oguz A, Cetiner S, Karadeniz C (1999) Disturbances in oral and dental structures in patients with pediatric lymphoma after chemotherapy: a preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 87:317–321
Bergmann OJ, Ellegaard B, Dahl M, Ellegaard J (1919) Gingival status during chemical plaque control with or without prior mechanical plaque removal in patients with acute myeloid leukaemia. J Clin Periodontol 19:169–173
Marques MA, Dib LL (2004) Periodontal changes in patients undergoing radiotherapy. J Periodontol 75:1178–1187
Toljanic JA, Bedard JF, Larson RA, Fox JP (1999) A prospective pilot study to evaluate a new dental assessment and treatment paradigm for patients scheduled to undergo intensive chemotherapy for cancer. Cancer 85:1843–1848
Laine PO, Lindqvist JC, Pyrhonen SO, Strand-Pettinen IM, Teerenhovi LM, Meurman JH (1992) Oral infection as a reason for febrile episodes in lymphoma patients receiving cytostatic drugs. Eur J Cancer B Oral Oncol 28B:103–107
Al-Joburi W, Clark C, Fisher R (1991) A comparison of the effectiveness of two systems for the prevention of radiation caries. Clin Prev Dent 13:15–19
Spak CJ, Johnson G, Ekstrand J (1994) Caries incidence, salivary flow rate and efficacy of fluoride gel treatment in irradiated patients. Caries Res 28:388–393
Meyerowitz C, Watson GE (1998) The efficacy of an intraoral fluoride-releasing system in irradiated head and neck cancer patients: a preliminary study. J Am Dent Assoc 129:1252–1259
Chambers MS, Fleming TJ, Toth BB, Lemon JC, Craven TE, Bouwsma OJ, Garden AS, Espeland MA, Keene HJ, Martin JW, Sipos T (2007) Erratum to “Clinical evaluation of the intraoral fluoride releasing system in radiation-induced xerostomic subjects. Part 2: phase I study”. Oral Oncol 43:98–105
Meurman JH, Laine P, Murtomaa H, Lindqvist C, Torkko H, Teerenhovi L, Pyrhonen S (1991) Effect of antiseptic mouthwashes on some clinical and microbiological findings in the mouths of lymphoma patients receiving cytostatic drugs. J Clin Periodontol 18:587–591
van Steenberghe D, Van den Eynde E, Jacobs R, Quirynen M (1994) Effect of a lactoperoxidase containing toothpaste in radiation-induced xerostomia. Int Dent J 44:133–138
Toljanic JA, Siddiqui AA, Patterson GL, Irwin ME (1996) An evaluation of a dentifrice containing salivary peroxidase elements for the control of gingival disease in patients with irradiated head and neck cancer. J Prosthet Dent 76:292–296
Papas A, Russell D, Singh M, Kent R, Triol C, Winston A (2008) Caries clinical trial of a remineralising toothpaste in radiation patients. Gerodontology 25:76–88
Joyston-Bechal S, Hayes K, Davenport ES, Hardie JM (1992) Caries incidence, mutans streptococci and lactobacilli in irradiated patients during a 12-month preventive programme using chlorhexidine and fluoride. Caries Res 26:384–390
McComb D, Erickson RL, Maxymiw WG, Wood RE (2002) A clinical comparison of glass ionomer, resin-modified glass ionomer and resin composite restorations in the treatment of cervical caries in xerostomic head and neck radiation patients [see comment]. Oper Dent 27:430–437
Wood RE, Maxymiw WG, McComb D (1993) A clinical comparison of glass ionomer (polyalkenoate) and silver amalgam restorations in the treatment of class 5 caries in xerostomic head and neck cancer patients. Oper Dent 18:94–102
Hu JY, Li YQ, Smales RJ, Yip KH (2002) Restoration of teeth with more-viscous glass ionomer cements following radiation-induced caries. Int Dent J 52:445–448
Rojas de Morales T, Zambrano O, Rivera L, Navas R, Chaparro N, Bernardonni C, Rivera F, Fonseca N, Tirado DM (2001) Oral-disease prevention in children with cancer: testing preventive protocol effectiveness. Med Oral 6:326–334
Rudat V, Meyer J, Momm F, Bendel M, Henke M, Strnad V, Grotz K, Schulte A (2000) Protective effect of amifostine on dental health after radiotherapy of the head and neck. Int J Radiat Oncol Biol Phys 48:1339–1343
Sela M, Gedalia I, Shah L, Skobe Z, Kashket S, Lewinstein I (1994) Enamel rehardening with cheese in irradiated patients. Am J Dent 7:134–136
Sela M, Maroz D, Gedalia I (2000) Streptococcus mutans in saliva of normal subjects and neck and head irradiated cancer subjects after consumption of honey. J Oral Rehabil 27:269–270
World Health Organization (1987) Oral health surveys: basic methods, 3rd edn. World Health Organization, Geneva
Becker T, Levin L, Shochat T, Einy S (2007) How much does the DMFT index underestimate the need for restorative care? J Dent Educ 71:677–681
Benigeri M, Payette M, Brodeur JM (1998) Comparison between the DMF indices and two alternative composite indicators of dental health. Community Dent Oral Epidemiol 26:303–309
Vanobbergen J, Martens L, Lesaffre E, Bogaerts K, Declerck D (2001) The value of a baseline caries risk assessment model in the primary dentition for the prediction of caries incidence in the permanent dentition. Caries Res 35:442–450
Epstein JB, Lunn R, Le N, Stevenson-Moore P (1998) Periodontal attachment loss in patients after head and neck therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:673–677
Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P, Calais G (2003) Late toxicity results of the GORTEC 94–01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG, EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys 55:93–98
Al-Nawas B, Grotz K (2006) Prospective study of the long term change of the oral flora after radiation therapy. Support Care Cancer 14:291–296
Roos DE, Dische S, Saunders MI (1996) The dental problems of patients with head and neck cancer treated with CHART. Eur J Cancer B Oral Oncol 32B:176–181
Epstein JB, van der Meij EH, Lunn R, Stevenson-Moore P (1996) Effects of compliance with fluoride gel application on caries and caries risk in patients after radiation therapy for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82:268–275
Almstahl A, Wikstrom M, Fagerberg-Mohlin B (2008) Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis 14:541–549
Epstein JB, Chin EA, Jacobson JJ, Rishiraj B, Le N (1998) The relationship among fluoride, cariogenic oral flora, and saliary flow rate during radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:286–292
Markitziu A, Zafiropoulos G, Tsalikis L, Cohen L (1992) Gingival health and salivary function in head and neck-irradiated patients. A five-year follow-up. Oral Surg Oral Med Oral Pathol 73:427–433
Leung WK, Jin LJ, Samaranayake LP, Chiu GK (1998) Subgingival microbiota of shallow periodontal pockets in individuals after head and neck irradiation. Oral Microbiol Immunol 13:1–10
Bakkal BH, Kaya B, Berberoglu S, Aksu G, Sayin MY, Altundag MB, Fayda M (2007) The efficiency of different chemoradiotherapy regimens in patients with paediatric nasopharynx cancer: review of 46 cases. Int J Clin Pract 61:52–61
O'Sullivan EA, Duggal MS, Bailey CC, Curzon MEJ, Hart P (1993) Changes in the oral microflora during cytotoxic chemotherapy in children being treated for acute leukemia. Oral Surg Oral Med Oral Pathol 76:161–168
Dens F, Boute P, Otten J, Vinckier F, Declerck D (1995) Dental caries, gingival health, and oral hygiene of long term survivors of paediatric malignant diseases. Arch Dis Child 72:129–132
Dens F, Boogaerts M, Boute P, Declerck D, Demuynck H, Vinckier F (1996) Caries-related salivary microorganisms and salivary flow rate in bone marrow recipients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:38–43
Dahllof G, Bagesund M, Ringden O (1997) Impact of conditioning regimens on salivary function, caries-associated microorganisms and dental caries in children after bone marrow transplantation. A 4-year longitudinal study. Bone Marrow Transplant 20:479–483
Jones LR, Toth BB, Keene HJ (1992) Effects of total body irradiation on salivary gland function and caries-associated oral microflora in bone marrow transplant patients. Oral Surg Oral Med Oral Pathol 73:670–676
Uderzo C, Fraschini D, Balduzzi A, Galimberti S, Arrigo C, Biagi E, Pignanelli M, Nicolini B, Rovelli A (1997) Long-term effects of bone marrow transplantation on dental status in children with leukaemia. Bone Marrow Transplant 20:865–869
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Conflict of interest statement
None to declare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hong, C.H.L., Napeñas, J.J., Hodgson, B.D. et al. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer 18, 1007–1021 (2010). https://doi.org/10.1007/s00520-010-0873-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0873-2