Abstract
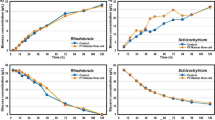
Multiple process analytical technology (PAT) tools are now being applied in tandem for cell culture. Research presented used two in-line probes, capacitance for a dynamic feeding strategy and Raman spectroscopy for real-time monitoring. Data collected from eight batches at the 15,000 L scale were used to develop process models. Raman spectroscopic data were modelled using Partial Least Squares (PLS) by two methods—(1) use of the full dataset and (2) split the dataset based on the capacitance feeding strategy. Root mean square error of prediction (RMSEP) for the first model method of capacitance was 1.54 pf/cm and the second modelling method was 1.40 pf/cm. The second Raman method demonstrated results within expected process limits for capacitance and a 0.01% difference in total nutrient feed compared to the capacitance probe. Additional variables modelled using Raman spectroscopy were viable cell density (VCD), viability, average cell diameter, and viable cell volume (VCV).








Similar content being viewed by others
References
Food and Drug Administration (2004) PAT—A Framework for Innovative Pharmaceutical Development, Manufac. In: U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pat-framework-innovative-pharmaceutical-development-manufacturing-and-quality-assurance. Accessed 30 Nov 2019
Mercier S, Diepenbroek B, Wijffels R, Streefland M (2014) Multivariate PAT solutions for biopharmaceutical cultivation: current progress and limitations. Trends Biotechnol 32:329–336. https://doi.org/10.1016/j.tibtech.2014.03.008
Zhang A, Tsang V, Moore B, Shen V, Huang Y, Kshirsagar R, Ryll T (2015) Advanced process monitoring and feedback control to enhance cell culture process production and robustness. Biotechnol Bioeng 112:2495–2504. https://doi.org/10.1002/bit.25684
Konakovsky V, Clemens C, Müller M, Bechmann J, Herwig C (2017) A robust feeding strategy to maintain set-point glucose in mammalian fed-batch cultures when input parameters have a large error. Biotechnol Prog 33:317–336. https://doi.org/10.1002/btpr.2438
Gagnon M, Hiller G, Luan Y, Kittredge A, DeFelice J, Drapeau D (2011) High-end pH-controlled delivery of glucose effectively suppresses lactate accumulation in CHO Fed-batch cultures. Biotechnol Bioeng 108:1328–1337. https://doi.org/10.1002/bit.23072
Berry B, Dobrowsky T, Timson R, Kshirsagar R, Ryll T, Wiltberger K (2015) Quick generation of Raman spectroscopy based in-process glucose control to influence biopharmaceutical protein product quality during mammalian cell culture. Biotechnol Prog 32:224–234. https://doi.org/10.1002/btpr.2205
Justice C, Brix A, Freimark D, Kraume M, Pfromm P, Eichenmueller B, Czermak P (2011) Process control in cell culture technology using dielectric spectroscopy. Biotechnol Adv 29:391–401. https://doi.org/10.1016/j.biotechadv.2011.03.002
Dowd J, Jubb A, Kwok K, Piret J (2003) Optimization and control of perfusion cultures using a viable cell probe and cell specific perfusion rates. Cytotechnology 42(1):35–45. https://doi.org/10.1023/A:1026192228471
Ansorge S, Esteban G, Schmid G (2010) On-line monitoring of responses to nutrient feed additions by multi-frequency permittivity measurements in fed-batch cultivations of CHO cells. Cytotechnology 62:121–132. https://doi.org/10.1007/s10616-010-9267-z
Opel C, Li J, Amanullah A (2010) Quantitative modeling of viable cell density, cell size, intracellular conductivity, and membrane capacitance in batch and fed-batch CHO processes using dielectric spectroscopy. Biotechnol Prog. https://doi.org/10.1002/btpr.425
Ma F, Zhang A, Chang D, Velev O, Wiltberger K, Kshirsagar R (2019) Real-time monitoring and control of CHO cell apoptosis by in situ multifrequency scanning dielectric spectroscopy. Process Biochem 80:138–145. https://doi.org/10.1016/j.procbio.2019.02.017
Lee H, Carvell J, Brorson K, Yoon S (2014) Dielectric spectroscopy-based estimation of VCD in CHO cell culture. J Chem Technol Biotechnol 90:273–282. https://doi.org/10.1002/jctb.4522
Downey B, Graham L, Breit J, Glutting N (2014) A novel approach for using dielectric spectroscopy to predict viable cell volume (VCV) in early process development. Biotechnol Prog 30:479–487. https://doi.org/10.1002/btpr.1845
Lu F, Toh P, Burnett I, Li F, Hudson T, Amanullah A, Li J (2012) Automated dynamic fed-batch process and media optimization for high productivity cell culture process development. Biotechnol Bioeng 110:191–205. https://doi.org/10.1002/bit.24602
Konakovsky V, Yagtu A, Clemens C, Müller M, Berger M, Schlatter S, Herwig C (2015) Universal capacitance model for real-time biomass in cell culture. Sensors 15:22128–22150. https://doi.org/10.3390/s150922128
Fernandes J, Currie J, Ramer K, Zhang A (2018) Development of capacitance tools: at-line method for assessing biomass of mammalian cell culture and fixed cell calibration standard. Biotechnol J 14:1800283. https://doi.org/10.1002/biot.201800283
Abu-Absi N, Kenty B, Cuellar M, Borys M, Sakhamuri S, Strachan D, Hausladen M, Li Z (2010) Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe. Biotechnol Bioeng 108:1215–1221. https://doi.org/10.1002/bit.23023
Capito F, Zimmer A, Skudas R (2015) Mid-infrared spectroscopy-based analysis of mammalian cell culture parameters. Biotechnol Prog 31:578–584. https://doi.org/10.1002/btpr.2026
Teixeira A, Portugal C, Carinhas N, Dias J, Crespo J, Alves P, Carrondo M, Oliveira R (2009) In situ 2D fluorometry and chemometric monitoring of mammalian cell cultures. Biotechnol Bioeng 102:1098–1106. https://doi.org/10.1002/bit.22125
Courtès F, Ebel B, Guédon E, Marc A (2016) A dual near-infrared and dielectric spectroscopies strategy to monitor populations of Chinese hamster ovary cells in bioreactor. Biotechnol Lett 38:745–750. https://doi.org/10.1007/s10529-016-2036-0
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst 58:109–130. https://doi.org/10.1016/s0169-7439(01)00155-1
Whelan J, Craven S, Glennon B (2012) In situ Raman spectroscopy for simultaneous monitoring of multiple process parameters in mammalian cell culture bioreactors. Biotechnol Prog 28:1355–1362. https://doi.org/10.1002/btpr.1590
Berry B, Moretto J, Matthews T, Smelko J, Wiltberger K (2014) Cross-scale predictive modeling of CHO cell culture growth and metabolites using Raman spectroscopy and multivariate analysis. Biotechnol Prog 31:566–577. https://doi.org/10.1002/btpr.2035
Mehdizadeh H, Lauri D, Karry K, Moshgbar M, Procopio-Melino R, Drapeau D (2015) Generic Raman-based calibration models enabling real-time monitoring of cell culture bioreactors. Biotechnol Prog 31:1004–1013. https://doi.org/10.1002/btpr.2079
André S, Lagresle S, Da Sliva A, Heimendinger P, Hannas Z, Calvosa É, Duponchel L (2017) Developing global regression models for metabolite concentration prediction regardless of cell line. Biotechnol Bioeng 114:2550–2559. https://doi.org/10.1002/bit.26368
Craven S, Whelan J, Glennon B (2014) Glucose concentration control of a fed-batch mammalian cell bioprocess using a nonlinear model predictive controller. J Process Control 24:344–357. https://doi.org/10.1016/j.jprocont.2014.02.007
Matthews T, Berry B, Smelko J, Moretto J, Moore B, Wiltberger K (2016) Closed loop control of lactate concentration in mammalian cell culture by Raman spectroscopy leads to improved cell density, viability, and biopharmaceutical protein production. Biotechnol Bioeng 113:2416–2424. https://doi.org/10.1002/bit.26018
Moore B, Sanford R, Zhang A (2019) Case study: the characterization and implementation of dielectric spectroscopy (biocapacitance) for process control in a commercial GMP CHO manufacturing process. Biotechnol Prog 35:e2782. https://doi.org/10.1002/btpr.2782
Cannizzaro C, Gügerli R, Marison I, von Stockar U (2003) On-line biomass monitoring of CHO perfusion culture with scanning dielectric spectroscopy. Biotechnol Bioeng 84:597–610. https://doi.org/10.1002/bit.10809
Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Vikström C, Wold S (2006) Multi- and megavariate data analysis. Umetrics, Umea
Ducommun P, Kadouri A, von Stockar U, Marison I (2001) On-line determination of animal cell concentration in two industrial high-density culture processes by dielectric spectroscopy. Biotechnol Bioeng 77:316–323. https://doi.org/10.1002/bit.1197
Acknowledgements
The authors thank the Manufacturing Operations and Manufacturing Sciences teams at the Biogen Hillerod site. A grateful thanks to the Irish Research Council for funding. Also, thanks to Barry McCarthy, Ronan Hayes and the Janssen department—BioTherapeutic Development.
Funding
Irish Research Council. Grant Number: EPSPG/2015/150.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rafferty, C., O’Mahony, J., Rea, R. et al. Raman spectroscopic based chemometric models to support a dynamic capacitance based cell culture feeding strategy. Bioprocess Biosyst Eng 43, 1415–1429 (2020). https://doi.org/10.1007/s00449-020-02336-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02336-2