Abstract
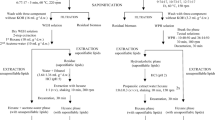
In recent years, there has been increasing consumer interest in carotenoids, particularly of marine sustainable origin with applications in the food, cosmeceutical, nutritional supplement and pharmaceutical industries. For instance, microalgae belonging to the genus Tetraselmis are known for their biotechnologically relevant carotenoid profile. The recently isolated marine microalgal strain Tetraselmis sp. CTP4 is a fast-growing, robust industrial strain, which has successfully been produced in 100-m3 photobioreactors. However, there are no reports on total carotenoid contents from this strain belonging to T. striata/convolutae clade. Although there are several reports on extraction methods targeting chlorophytes, extraction depends on the strength of cell coverings, solvent polarity and the nature of the targeted carotenoids. Therefore, this article evaluates different extraction methods targeting Tetraselmis sp. CTP4, a strain known to contain a mechanically resistant theca. Here, we propose a factorial experimental design to compare extraction of total carotenoids from wet and freeze-dried microalgal biomass using four different solvents (acetone, ethanol, methanol or tetrahydrofuran) in combination with two types of mechanical cell disruption (glass beads or dispersion). The extraction efficiency of the methods was assessed by pigment contents and profiles present in the extracts. Extraction of wet biomass by means of glass bead-assisted cell disruption using tetrahydrofuran yielded the highest amounts of lutein and β-carotene (622 ± 40 and 618 ± 32 µg g−1 DW, respectively). Although acetone was slightly less efficient than tetrahydrofuran, it is preferable due to its lower costs and toxicity.




Similar content being viewed by others
References
Varela JC, Pereira H, Vila M, León R (2015) Production of carotenoids by microalgae: achievements and challenges. Photosynth Res 125:423–436. https://doi.org/10.1007/s11120-015-0149-2
Johnson EJ (2002) The role of carotenoids in human health. Nutr Clin Care 5:56–65. https://doi.org/10.1046/j.1523-5408.2002.00004.x
Fiedor J, Burda K (2014) Potential role of carotenoids as antioxidants in human health and disease. Nutrients 6:466–488. https://doi.org/10.3390/nu6020466
Eggersdorfer M, Wyss A (2018) Carotenoids in human nutrition and health. Arch Biochem Biophys 652:18–26. https://doi.org/10.1016/j.abb.2018.06.001
Vílchez C, Forján E, Cuaresma M, Bédmar F, Garbayo I, Vega JM (2011) Marine carotenoids: biological functions and commercial applications. Mar Drugs 9:319–333. https://doi.org/10.3390/md9030319
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644. https://doi.org/10.3390/md9040625
Borowitzka MA (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25:743–756. https://doi.org/10.1007/s10811-013-9983-9
Sánchez JF, Fernández JM, Acién FG, Rueda A, Pérez-Parra J, Molina E (2008) Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem 43:398–405. https://doi.org/10.1016/j.procbio.2008.01.004
Ben-Amotz A, Shaish A, Avron M (1989) Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol 91:1040–1043. https://doi.org/10.1104/pp.91.3.1040
Mulders KJM, Lamers PP, Martens DE, Wijffels RH (2014) Phototrophic pigment production with microalgae: biological constraints and opportunities. J Phycol 50:229–242. https://doi.org/10.1111/jpy.12173
Siegel BZ, Siegel SM (1973) The chemical composition of algal cell walls. CRC Crit Rev Microbiol 3:1–26. https://doi.org/10.3109/10408417309108743
Becker B, Melkonian M, Kamerling JP (1998) The cell wall (theca) of Tetraselmis striata (chlorophyta): Macromolecular composition and structural elements of the complex polysaccharides. J Phycol 34:779–787. https://doi.org/10.1046/j.1529-8817.1998.340779.x
Fernández-Sevilla JM, Fernández AFG, Grima ME (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86:27–40. https://doi.org/10.1007/s00253-009-2420-y
Saini RK, Keum YS (2018) Carotenoid extraction methods: a review of recent developments. Food Chem 240:90–103. https://doi.org/10.1016/j.foodchem.2017.07.099
Arvayo-Enríquez H, Mondaca-Fernández I, Gortárez-Moroyoqui P, López-Cervantes J, Rodríguez-Ramírez R (2013) Carotenoids extraction and quantification: a review. Anal Methods 5:2916–2924. https://doi.org/10.1039/c3ay26295b
Pereira H, Gangadhar KN, Schulze PSC, Santos T, de Sousa CB, Schueler LM, Custódio L, Malcata FX, Gouveia L, Varela JCS, Barreira L (2016) Isolation of a euryhaline microalgal strain, Tetraselmis sp. CTP4, as a robust feedstock for biodiesel production. Sci Rep 6:35663. https://doi.org/10.1038/srep35663
Pereira H, Páramo J, Silva J, Marques A, Barros A, Maurício D, Santos T, Schulze P, Barros R, Gouveia L, Barreira L, Varela J (2018) Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: from an agar plate to 100–m3 industrial photobioreactors. Sci Rep 8:5112. https://doi.org/10.1038/s41598-018-23340-3
Schulze PSC, Carvalho CFM, Pereira H, Gangadhar KN, Schüler LM, Santos TF, Varela JCS, Barreira L (2017) Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour Technol 223:175–183. https://doi.org/10.1016/j.biortech.2016.10.027
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Couso I, Vila M, Vigara J, Cordero BF, Vargas MÁ, Rodríguez H, León R (2012) Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. Eur J Phycol 47:223–232. https://doi.org/10.1080/09670262.2012.692816
del Cerón-García M, Campos-Pérez I, Macías-Sánchez MD, Bermejo-Román R, Fernández-Sevilla JM, Molina-Grima E (2010) Stability of carotenoids in Scenedesmus almeriensis biomass and extracts under various storage conditions. J Agric Food Chem 58:6944–6950. https://doi.org/10.1021/jf100020s
Ryckebosch E, Muylaert K, Eeckhout M, Ruyssen T, Foubert I (2011) Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J Agric Food Chem 59:11063–11069. https://doi.org/10.1021/jf2025456
Taucher J, Baer S, Schwerna P, Hofmann D, Hümmer M, Buchholz R, Becker A (2016) Cell disruption and pressurized liquid extraction of carotenoids from microalgae. Thermodyn Catal 7:1–7. https://doi.org/10.4172/2158-7544.1000158
Hu CW, Te CL, Yu PC, Chen CNN (2013) Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem 138:2071–2078. https://doi.org/10.1016/j.foodchem.2012.11.133
Tsai H-P, Chuang L-T, Chen C-NN (2016) Production of long chain omega-3 fatty acids and carotenoids in tropical areas by a new heat-tolerant microalga Tetraselmis sp. DS3. Food Chem 192:682–690. https://doi.org/10.1016/j.foodchem.2015.07.071
Goiris K, Van Colen W, Wilches I, León-Tamariz F, De Cooman L, Muylaert K (2015) Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res 7:51–57. https://doi.org/10.1016/j.algal.2014.12.002
Garrido JL, Rodríguez F, Zapata M (2009) Occurence of loroxanthin, loroxanthin decenoate, and loroxanthin dodecenoate in Tetraselmis species (Prasinophyceae, Chlorophyta). J Phycol 45:366–374. https://doi.org/10.1111/j.1529-8817.2009.00660.x
Wright SW, Jeffrey SW, Mantoura RFC (1997) Evaluation of methods and solvents for pigment extraction. In: Jeffrey SW, Wright SW, Mantoura RFC (eds) Phytoplankton pigments in oceanogpaphy: guidelines to modern methods. UNESCO Publishing, Paris, pp 261–282
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a. Mar Ecol Prog Ser 195:29–45. https://doi.org/10.3354/meps195029
Di Lena G, Casini I, Lucarini M, Lombardi-Boccia G (2019) Carotenoid profiling of five microalgae species from large-scale production. Food Res Int 120:810–818. https://doi.org/10.1016/j.foodres.2018.11.043
Petrier C, Jeunet A, Luche JL, Reverdy G (1992) Unexpected frequency effects on the rate of oxidative processes induced by ultrasound. J Am Chem Soc 114:3148–3150. https://doi.org/10.1021/ja00034a077
Geciova J, Bury D, Jelen P (2002) Methods for disruption of microbial cells for potential use in the dairy industry: a review. Int Dairy J 12:541–553. https://doi.org/10.1016/S0958-6946(02)00038-9
Schwenzfeier A, Wierenga PA, Gruppen H (2011) Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour Technol 102:9121–9127. https://doi.org/10.1016/j.biortech.2011.07.046
Spiden EM, Yap BHJ, Hill DRA, Kentish SE, Scales PJ, Martin GJO (2013) Bioresource technology quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour Technol 140:165–171. https://doi.org/10.1016/j.biortech.2013.04.074
Khachik F, Beecher GR, Whittaker NF (1986) Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. J Agric Food Chem 34:603–616. https://doi.org/10.1021/jf00070a006
Rivera S, Canela R (2012) Influence of sample processing on the analysis of carotenoids in maize. Molecules 17:11255–11268. https://doi.org/10.3390/molecules170911255
van Leeuwe MA, Villerius LA, Roggeveld J, Visser RJW, Stefels J (2006) An optimized method for automated analysis of algal pigments by HPLC. Mar Chem 102:267–275. https://doi.org/10.1016/j.marchem.2006.05.003
Zapata M, Garrido JL (1991) Influence of injection conditions in reversed-phase high-performance liquid chromatography of chlorophylls and carotenoids. Chromatographia 31:589–594. https://doi.org/10.1007/BF02279480
Wright S, Jeffrey S, Mantoura R, Llewellyn C, Bjornland T, Repeta D, Welschmeyer N (1991) Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar Ecol Prog Ser 77:183–196. https://doi.org/10.3354/meps077183
Porra RJ, Pfündel EE, Engel N (1997) Metabolism and function of photosynthetic pigments. In: Jeffrey SW, Wright SW, Mantoura RFC (eds) Phytoplankton pigments in oceanogpaphy: guidelines to modern methods. UNESCO Publishing, Paris, pp 85–126
Ahmed F, Fanning K, Netzel M, Turner W, Li Y, Schenk PM (2014) Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem 165:300–306. https://doi.org/10.1016/j.foodchem.2014.05.107
Craft NE, Soares JH (1992) Relative solubility, stability, and absorptivity of lutein and β-carotene in organic solvents. J Agric Food Chem 40:431–434. https://doi.org/10.1021/jf00015a013
Chen CY, Jesisca HC, Lee DJ, Chang CH, Chang JS (2016) Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresour Technol 200:500–505. https://doi.org/10.1016/j.biortech.2015.10.071
Efsa ANS (2012) Scientific opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J 10:2588. https://doi.org/10.2903/j.efsa.2012.2588
Goiris K, Muylaert K, Fraeye I, Foubert I, De Brabanter J, De Cooman L (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol 24:1477–1486. https://doi.org/10.1007/s10811-012-9804-6
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2:164–173. https://doi.org/10.1016/j.algal.2013.01.004
Mulders KJM, Weesepoel Y, Bodenes P, Lamers PP, Vincken J, Martens DE, Gruppen H, Wijffels RH (2015) Nitrogen-depleted Chlorella zofingiensis produces astaxanthin, ketolutein and their fatty acid esters: a carotenoid metabolism study. J Appl Phycol 27:125–140. https://doi.org/10.1007/s10811-014-0333-3
León R, Vila M, Hernánz D, Vílchez C (2005) Production of phytoene by herbicide-treated microalgae Dunaliella bardawil in two-phase systems. Biotechnol Bioeng 92:695–701. https://doi.org/10.1002/bit.20660
Lamers PP, van de Laak CCW, Kaasenbrood PS, Lorier J, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648. https://doi.org/10.1002/bit.22725
Huang JJ, Bunjamin G, Teo ES, Ng DB, Lee YK (2016) An enclosed rotating floating photobioreactor (RFP) powered by flowing water for mass cultivation of photosynthetic microalgae. Biotechnol Biofuels 9:218. https://doi.org/10.1186/s13068-016-0633-8
Castro-Puyana M, Herrero M, Urreta I, Mendiola JA, Cifuentes A, Ibáñez E, Suárez-Alvarez S (2013) Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 405:4607–4616. https://doi.org/10.1007/s00216-012-6687-y
Lourenço SO, Marquez UML, Mancini-Filho J, Barbarino E, Aidar E (1997) Changes in biochemical profile of Tetraselmis gracilis I. Comparison of two culture media. Aquaculture 148:153–168. https://doi.org/10.1016/S0044-8486(96)01416-0
Dahmen-Ben Moussa I, Chtourou H, Karray F, Sayadi S, Dhouib A (2017) Nitrogen or phosphorus repletion strategies for enhancing lipid or carotenoid production from Tetraselmis marina. Bioresour Technol 238:325–332. https://doi.org/10.1016/j.biortech.2017.04.008
Sansone C, Galasso C, Orefice I, Nuzzo G, Luongo E, Cutignano A, Romano G, Brunet C, Fontana A, Esposito F, Ianora A (2017) The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Natl Publ. https://doi.org/10.1038/srep41215
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D’Ottavio M, Tredici MR (2014) Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M-M33 grown with LEDs of different colors. Biotechnol Bioeng 111:956–964. https://doi.org/10.1002/bit.25014
Borghini F, Colacevich A, Bergamino N, Micarelli P, Dattilo AM, Focardi S, Focardi S, Loiselle SA (2009) The microalgae Tetraselmis suecica in mesocosms under different light regimes. Chem Ecol 25:345–357. https://doi.org/10.1080/02757540903193148
El-Kassas HY, El-Sheekh MM (2016) Induction of the synthesis of bioactive compounds of the marine alga Tetraselmis tetrathele (West) Butcher grown under salinity stress. Egypt J Aquat Res 42:385–391. https://doi.org/10.1016/j.ejar.2016.10.006
Egeland ES, Eikrem W, Throndsen J, Wilhelm C, Zapata M, Liaaen-Jensen S (1995) Carotenoids from further prasinophytes. Biochem Syst Ecol 23:747–755. https://doi.org/10.1016/0305-1978(95)00075-5
Dammak M, Hadrich B, Miladi R, Barkallah M, Hentati F, Hachicha R, Laroche C, Michaud P, Fendri I, Abdelkafi S (2017) Effects of nutritional conditions on growth and biochemical composition of Tetraselmis sp. Lipids Health Dis 16:41. https://doi.org/10.1186/s12944-016-0378-1
Maadane A, Merghoub N, Ainane T, El Arroussi H, Benhima R, Amzazi S, Bakri Y, Wahby I (2015) Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J Biotechnol 215:13–19. https://doi.org/10.1016/j.jbiotec.2015.06.400
Funding
The authors are indebted to the Foundation for Science and Technology (FCT), Portugal, for funding through UID/Multi/04326/2019 research program and a doctoral research grant (SFRH/BD/115325/2016) awarded to LS. Further funding was provided by the 0055 ALGARED + 05 INTERREG V-A–España Portugal project. KNG also is thankful to the FCT and University of Algarve for financial support under the transitional rule of Decree-Law no. 57/2016 as amended by Law No 57/2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schüler, L.M., Gangadhar, K.N., Duarte, P. et al. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess Biosyst Eng 43, 785–796 (2020). https://doi.org/10.1007/s00449-019-02273-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02273-9