Abstract
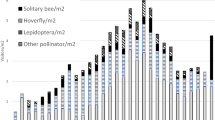
Parasitic plants often attack multiple host species with unique defenses, physiology, and ecology. Reproductive phenology and vectors of parasitic plant genes (pollinators and dispersers) can contribute to or erode reproductive isolation of populations infecting different host species. We asked whether desert mistletoe, Phoradendron californicum (Santalaceae tribe Visceae syn. Viscaceae), differs ecologically across its dominant leguminous hosts in ways affecting reproductive isolation. Parasite flowering phenology on one host species (velvet mesquite, Prosopis velutina) differed significantly from that on four others, and phenology was not predicted by host species phenology or host individual. Comparing mistletoe populations on mesquite and another common host species (catclaw acacia, Senegalia greggii) for which genetically distinct host races are known, we tested for differences in interactions with vectors by quantifying pollinator visitation, reward production, pollen receipt, and fruit consumption. Mistletoes on mesquite produced more pollinator rewards per flower (1.86 times the nectar and 1.92 times the pollen) and received ~ 2 more pollen grains per flower than those on acacia. Mistletoes on the two host species interacted with distinct but overlapping pollinator communities, and pollinator taxa differed in visitation according to host species. Yet, mistletoes of neither host showed uniformly greater reproductive success. Fruit set (0.70) did not differ by host, and the rates of fruit ripening and removal differed in contrasting ways. Altogether, we estimate strong but asymmetric pre-zygotic isolating barriers between mistletoes on the two hosts. These host-associated differences in reproduction have implications for interactions with mutualist vectors and population genetic structure.




Similar content being viewed by others
References
Altizer SM, Thrall PH, Antonovics J (1998) Vector behavior and the transmission of anther-smut infection in Silene alba. Am Midl Nat 139:147–163. https://doi.org/10.1674/0003-0031(1998)139[0147:vbatto]2.0.co;2
Aukema JE (2002) Variation in mistletoe seed deposition: effects of intra- and interspecific host characteristics. Ecography 25:139–144
Aukema JE (2003) Vectors, viscin, and Viscaceae: mistletoes as parasites, mutualists, and resources. Front Ecol Environ 1:212–219
Aukema JE (2004) Distribution and dispersal of desert mistletoe is scale-dependent, hierarchically nested. Ecography 27:137–144
Brody AK (1997) Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology 78:1624–1631
Burgess TL (1995) Desert grassland, mixed shrub savanna, shrub steppe, or semidesert scrub? The dilemma of coexisting growth forms. In: McClaran MP, Van Devender TR (eds) The desert grassland. Univ. of Arizona Press, USA, pp 31–64
Calero-Torralbo M, Valera (2008) Synchronization of host-parasite cycles by means of diapause: Host influence and parasite response to involuntary host shifting. Parasitol 135:1343–1352
Candia AB, Medel R, Fonturbel FE (2014) Indirect positive effects of a parasitic plant on host pollination and seed dispersal. Oikos 123:1371–1376
Caraballo-Ortiz MA, Gonzalez-Castro A, Yang S, dePamphilis CW, Carlo TA (2017) Dissecting the contributions of dispersal and host properties to the local abundance of a tropical mistletoe. J Ecol 105:1657–1667
Clark RM, Thompson R (2011) Estimation and comparison of flowering curves. Plant Ecol Divers 4:189–200. https://doi.org/10.1080/17550874.2011.580382
Clay K, Dement D, Rejmanek M (1985) Experimental evidence for host races in Mistletoe (Phoradendron tomentosum). Am J Bot 72:1225–1231
Craig TP, Horner JD, Itami JK (1997) Hybridization studies on the host races of Eurosta solidaginis: implications for sympatric speciation. Evol 51:1552–1560. https://doi.org/10.1111/j.1558-5646.1997.tb01478.x
De Vega C, Berjano R, Arista M, Ortiz PL, Talavera S, Stuessy TF (2008) Genetic races associated with the genera and sections of host species in the holoparasitic plant Cytinus (Cytinaceae) in the Western Mediterranean basin. New Phytol 178:875–887. https://doi.org/10.1111/j.1469-8137.2008.02423.x
De Vienne DM, Refrégier G, López-Villavicencio M, Tellier A, Hood ME, Giraud T (2013) Cospeciation vs host-shift speciation: methods for testing, evidence from natural associations and relation to coevolution. New Phytol 198:347–385. https://doi.org/10.1111/nph.12150
Drès M, Mallet J (2002) Host races in plant-feeding insects and their importance in sympatric speciation. Philos Trans R Soc Lond B Biol Sci 357:471–492. https://doi.org/10.1098/rstb.2002.1059
Feder JL, Filchak KE (1999) It’s about time: The evidence for host plant-mediated selection in the apple maggot fly, Rhagoletis pomonella, and its implications for fitness trade-offs in phytophagous insects. Entomol Exp Appl 91:211–225. https://doi.org/10.1023/A:1003603918154
Ferrari J, Godfray HCJ, Faulconbridge AS, Prior K, Via S (2006) Population differentiation and genetic variation in host choice among pea aphid from eight host plant genera. Evolution 60:1574–1584
Gaddis KD (2014) The population biology of dispersal and gene flow in the desert shrub Acacia (Senegalia) greggii A. Gray in the Mojave National Preserve. In: Ph.D. thesis, University of California, Los Angeles, CA
Gibson CC, Watkinson AR (1989) The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia 78:401–406
Glazner JT, Devhn B, Ellstrand NC (1988) Biochemical and morphological evidence for host race evolution in desert mistletoe, Phoradendron californicum (Viscaceae). Plant Syst Evol 161:13–21
Hopkins R (2013) Reinforcement in plants. New Phytol 197:1095–1103. https://doi.org/10.1111/nph.12119
Jerome CA, Ford BA (2002) The discovery of three genetic races of the dwarf mistletoe Arceuthobium americanum (Viscaceae) provides insight into the evolution of parasitic angiosperms. Mol Ecol 11:387–405
Kahle-Zuber D (2008) Biology and evolution of the European mistletoe (Viscum album). In: Ph.D thesis, ETH Zurich, Zurich, Switzerland
Kelly D, Ladley JJ, Robertson AW (2007) Is the pollen-limited mistletoe Peraxilla tetrapetala (Loranthaceae) also seed limited? Aust Ecol 32:850–857
Keys R, Buchmann S, Smith S (1995) Pollination effectiveness and pollination efficiency of insects foraging Prosopis velutina in south-eastern Arizona. J Appl Ecol 32:519–527. https://doi.org/10.2307/2404649
Kiss L, Pintye A, Kovacs GM, Jankovics T, Fontaine MC, Harvey N, Xu X, Nicot PC, Bardin M, Shykoff JA, Giraud T (2011) Temporal isolation explains host-related genetic differentiation in a group of widespread mycoparasitic fungi. Mol Ecol 20:1492–1507
Komatsu T, Akimoto S (1995) Genetic differentiation as a result of adaptation to the phenologies of individual host trees in the galling aphid Kaltenbachiella japonica. Ecol Entomol 20:33–42
Ladley JJ, Kelly D (1996) Dispersal, germination, and survival of New Zealand mistletoes (Loranthaceae): dependence on birds. NZ J Ecol 20:69–79
Ladley JJ, Kelly D, Robertson AW (1997) Explosive flowering, nectar production, breeding systems, and pollinators of New Zealand mistletoes (Loranthaceae). NZ J Bot 35:345–360
Lafferty KD, Dobson AP, Kuris AM (2006) Parasites dominate food web links. Proc Natl Acad Sci USA 103:11211–11216. https://doi.org/10.1073/pnas.0604755103
Larson DL (1991) Ecology of desert mistletoe seed dispersal. In: Ph.D thesis, University of Illinois at Chicago, Chicago, IL
Larson DL (1996) Seed dispersal by specialist versus generalist foragers: the plant’s perspective. Oikos 76:113–120
Le Gac M, Giraud T (2004) What is sympatric speciation in parasites? Trend Parasitol 20:207–208. https://doi.org/10.1016/j.pt.2004.03.005
Li J, Corajod J, Deyoung J (2010) Host preferences of beechdrops (Epifagus): evidence from chloroplast DNA sequence data. Mich Bot 49:79–84
Lichter JM, Berry AM (1991) Establishment of the mistletoe Phoradendron macrophyllum: phenology of early stages and host compatibility studies. Bot Gaz 146:468–475
Linn C, Feder JL, Nojima S, Dambroski HR, Berlocher SH, Roelofs W (2003) Fruit odor discrimination and sympatric host race formation in Rhagoletis. Proc Natl Acad Sci USA 100:11490–11493. https://doi.org/10.1073/pnas.1635049100
Lira-Noriega A, Toro-Nunez O, Oaks JR, Mort ME (2015) The roles of history and ecology in chloroplast phylogeographic patterns of the bird-dispersed plant parasite Phoradendron californicum (Viscaceae) in the Sonoran Desert. Am J Bot 102:149–164
Marquardt ES, Pennings SC (2010) Constraints on host use by a parasitic plant. Oecol 164:177–184
Mattsson M, Hood GR, Feder JL, Ruedas LA (2015) Rapid and repeatable shifts in life-history timing of Rhagoletis pomonella (Diptera: Tephritidae) following colonization of novel host plants in the Pacific Northwestern United States. Ecol Evol. https://doi.org/10.1002/ece3.1826
Nickrent DL (2002) Mistletoe phylogenetics: Current relationships gained from analysis of DNA sequences. In: Proceedings of the Western International Forest Disease Work Conference, August 14–18, 2000. Waikoloa, Hawai’i, pp 48–57
Nickrent DL (2011) Santalales (including mistletoes). Encyclopedia of Life Sciences. doi:https://doi.org/10.1002/9780470015902.a0003714.pub2
Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, de Pamphilis CW (1998) Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II. Kluwer, Boston, pp 211–241
Norton DA, Carpenter MA (1998) Mistletoes as parasites: host specificity and speciation. Trend Ecol Evol 5347:101–105
Norton DA, De Lange PJ (1999) Host specificity in parasitic mistletoes (Loranthaceae) in New Zealand. Funct Ecol 13:552–559
Ollerton J, Stott A, Allnutt E, Shove S, Taylor C, Lamborn E (2007) Pollination niche overlap between a parasitic plant and its host. Oecologia 151:473–485. https://doi.org/10.1007/s00442-006-0605-y
Overton JM (1997) Host specialization and partial reproductive isolation in desert mistletoe (Phoradendron californicum). Southwest Nat 42:201–209
Rathcke B (1983) Competition and facilitation among plants for pollination. In: Real L (ed) Pollination biology. Academic Press, London, pp 305–329
Restrepo C, Sargent S, Levey D, Watson D (2002) The role of vertebrates in the diversification of New World mistletoes. In: Galetti MGM (ed) Seed dispersal and frugivory: ecology, evolution and conservation, 6th edn. CABI Publishing, Wallingford, UK, pp 83–98
Robertson AW, Kelly D, Ladley JJ, Sparrow AD (1999) Effects of pollinator loss on endemic New Zealand mistletoes (Loranthaceae). Cons Bio 13:499–508
Roxburgh L, Nicolson SW (2005) Patterns of host use in two African mistletoes: the importance of mistletoe-host compatibility and avian disperser behavior. Funct Ecol 19:865–873
Schulze ED, Ehleringer JR (1984) The effect of nitrogen supply on growth and water-use efficiency of xylem-tapping mistletoes. Planta 162:268–275
Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA (2012) Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc Biol Sci 279:925–933. https://doi.org/10.1098/rspb.2011.1282
Sobel JM, Chen GF (2014) Unification of methods for estimating the strength of reproductive isolation. Evol 68:1511–1522
Thurgood CJ, Rumsey FJ, Harris SA, Hiscock SJ (2008) Host-driven divergence in the parasitic plant Orobanche minor Sm. (Orobanchaceae). Mol Ecol 17:4289–4303
USA National Phenology Network (2015) Plant phenology data for the United States, 2011-01 to 2015-07. In: USA-NPN, Tucson, Arizona, USA
van Dongen S, Backeljau T, Matthysen E, Dhondt AA (1997) Synchronization of hatching date with budburst of individual host trees (Quercus robur) in the winter moth (Operophtera brumata) and its fitness consequences. J Anim Ecol 66:113–121. https://doi.org/10.2307/5969
van Ommeren RJ, Whitham TG (2002) Changes in interactions between juniper and mistletoe mediated by shared avian frugivores: parasitism to potential mutualism. Oecologia 130:281–288. https://doi.org/10.1007/s004420100792
Walsberg GE (1975) Digestive adaptations of Phainopepla nitens associated with the eating of mistletoe berries. Condor 77:169–174
Walsberg GE (1977) Ecology and energetics of contrasting social systems in Phainopepla nitens (Aves: Ptilogonatidae). University of California Press, Oakland
Wiens JJ, Lapoint RT, Whiteman NK (2015) Herbivory increases diversification across insect clades. Nat Commun 6:8370. https://doi.org/10.1038/ncomms9370
Wiesenborn WD (2016) Conspecific pollen loads on insects visiting female flowers on parasitic Phoradendron californicum (Viscaceae). West North Am Nat 76:113–121
Yule KM, Koop JAH, Alexandre NM, Johnston LR, Whiteman NK (2016) Population structure of a vector-borne plant parasite. Mol Ecol 25:3332–3343
Zitzer SF, Archer SR, Boutton TW (1996) Spatial variability in the potential for symbiotic N2 fixation by woody plants in a subtropical savanna ecosystem. J Appl Ecol 33:1125–1136
Zuber D, Widmer A (2009) Phylogeography and host race differentiation in the European mistletoe (Viscum album L.). Mol Ecol 18:1946–1962
Acknowledgements
We thank J. Knighton/Wisor, M. A. Iacuelli, A. L. Pond, E. May, and J. P. Berry for help with fieldwork and the Bronstein lab group for helpful comments. We acknowledge support from the following: a National Science Foundation (NSF) Doctoral Dissertation Improvement Grant to KMY and JLB (DEB-1601370), a Graduate Research Fellowship to KMY (DGE-1143953), a Ginny Saylor Research Grant from the Arizona Native Plants Society to KMY, and a University of Arizona Graduate Research and Project Grant to KMY.
Author information
Authors and Affiliations
Contributions
KMY and JLB conceived the study. KMY conducted the field work and data analyses. KMY and JLB wrote the manuscript.
Corresponding author
Additional information
Communicated by Amy Parachnowitsch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yule, K.M., Bronstein, J.L. Reproductive ecology of a parasitic plant differs by host species: vector interactions and the maintenance of host races. Oecologia 186, 471–482 (2018). https://doi.org/10.1007/s00442-017-4038-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-4038-6