Abstract
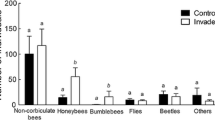
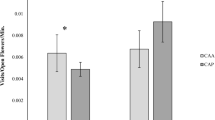
The structural organization of mutualism networks, typified by interspecific positive interactions, is important to maintain community diversity. However, there is little information available about the effect of introduced species on the structure of such networks. We compared uninvaded and invaded ecological communities, to examine how two species of invasive plants with large and showy flowers (Carpobrotus affine acinaciformis and Opuntia stricta) affect the structure of Mediterranean plant–pollinator networks. To attribute differences in pollination to the direct presence of the invasive species, areas were surveyed that contained similar native plant species cover, diversity and floral composition, with or without the invaders. Both invasive plant species received significantly more pollinator visits than any native species and invaders interacted strongly with pollinators. Overall, the pollinator community richness was similar in invaded and uninvaded plots, and only a few generalist pollinators visited invasive species exclusively. Invasive plants acted as pollination super generalists. The two species studied were visited by 43% and 31% of the total insect taxa in the community, respectively, suggesting they play a central role in the plant–pollinator networks. Carpobrotus and Opuntia had contrasting effects on pollinator visitation rates to native plants: Carpobrotus facilitated the visit of pollinators to native species, whereas Opuntia competed for pollinators with native species, increasing the nestedness of the plant–pollinator network. These results indicate that the introduction of a new species to a community can have important consequences for the structure of the plant–pollinator network.



Similar content being viewed by others
References
Aigner PA (2004) Ecological and genetic effects on demographic processes: pollination, clonality and seed production in Dithyrea maritima. Biol Conserv 116:27–34
Bascompte J, Jordano P, Melián CJ, Olesen J (2003) The nested assembly of plant–animal mutualistic networks. Proc Natl Acad Sci USA 100:9383–9387
Bascompte J, Jordano P, Olesen J (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312:431–433
Bascompte J, Jordano P (2007) Plant–animal mutualistic networks: the archictecture of biodiversity. Annu Rev Ecol Evol Syst 38:567–593
Bjerknes AL, Totland O, Hegland SJ, Nielsen A (2007) Do alien plant invasions really affect pollination success in native plant species? Biol Conserv 138:1–12
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (Purple loosestrife) and a native congener. Ecology 83:2328–2336
Campbell DR (1989) Inflorescence size: test of the male function hypothesis. Am J Bot 76:730–738
Chittka L, Schürkens S (2001) Successful invasion of a floral market. Nature 411:653–653
Devoto M, Medan D, Montaldo NH (2005) Patterns of interaction between plants and pollinators along an environmental gradient. Oikos 109:461–472
Dukas R, Bernays E A (2001) Learning improves growth rate in grasshoppers. Proc Natl Acad Sci USA 97:2637–2640
Fleishman E, Mac Nally R, Murphy D D (2005) Relationships among non-native plants, diversity of plants and butterflies, and adequacy of spatial sampling. Biol J Linn Soc 85:157–166
Graves SD, Shapiro AM (2003) Exotics as host plants of the California butterfly fauna. Biol Conserv 110:413–433
Guimaraes PR Jr, Guimaraes P (2006) Improving the analyses of nestedness for large sets of matrices. Environ Model Softw 21:1512–1513
Jordano P (1987) Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Am Nat 129:657–677
Jordano P, Bascompte J, Olesen J (2003) Invariant properties in coevolutionary networks of plant–animal interactions. Ecol Lett 6:69–81
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112
Kevan PG, Baker HG (1983) Insects as flower visitors and pollinators. Annu Rev Entomol 28:407–453
Kunin WE (1997) Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. J Ecol 85:225–234
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Griguils K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond 270:775–781
Lockwood JL, McKinney ML (2001) Biotic homogenization. New York Press, New York
Lodge DM (1993) Biological invasions—lessons for ecology. Trends Ecol Evol 8:133–137
Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J (2007) The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecol Lett 10:539–550
Memmott J, Wasser NM (2002) Integration of alien plants into a native flower-pollinator visitation web. Proc R Soc Lond 269:2395–2399
Michener CD (2000) Bees of the world. Johns Hopkins University Press, Baltimore
Olesen J, Eskildsen LI, Venkatasami S (2002) Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic spear generalists. Diver Distr 8:181–192
Olesen J, Jordano P (2002) Geographic patterns in plant-pollinator mutualistic networks. Ecology 83:2416–2424
Ollerton J, Cranmer L (2002) Latitudinal trends in plant-pollinator interactions: are tropical plants more specialised? Oikos 98:340–345
Petanidou T, Ellis WN (1993) Pollinating fauna of a phryganic ecosystem: composition and diversity. Biodiv Lett 1:9–22
Petanidou T, Lamborn E (2005) A land for flowers and bees: studying pollination ecology in Mediterranean communities. Plant Biosyst 139:279–294
Richardson DM, Allsopp N, D’Antonio CM (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Richardson DM, Pyšek P (2000) Naturalization and invasion of alien plants: concepts and definitions. Diver Distr 6:93–107
Sahli HF, Conner JK (2006) Characterizing ecological generalization in plant-pollination systems. Oecologia 148:365–372
Sanz-Elorza M, Dana ED, Sobrino D (2006) Atlas de las plantas alóctonas invasoras de España. Dirección General para la Biodiversidad, Madrid
SAS (2001) SAS (data analysis software system), version 9. SAS institute, Cary
Shmida A, Dafni A (1989) Blooming strategies, flower size and advertising in the “lily-group” geophytes in Israel. Herbertia 45:111–123
StatSoft (2001) STATISTICA (data analysis software system), version 6. Statsoft, Tulsa
Steffan-Dewenter I, Potts SG, Packer L (2005) Pollinator diversity and crop pollination services are at risk. Trends Ecol Evol 20:651–652
Steffan-Dewenter I, Tscharntke T (2001) Succession of bee communities on fallows. Ecography 24:83–93
Stout JC, Parnell JAN, Arroyo J, Crowe TP (2006) Pollination ecology and seed production of Rhododendron ponticum in native and exotic habitats. Biodivers Conserv 15:755–777
Suehs CM, Affre L, Médail F (2004) Invasion dynamics of two alien Carpobrotus taxa on a Mediterranean island. Heredity 92:550–556
Traveset A, Moragues E (2005) Effect of Carpobrotus spp. on the pollination success of native plant species of the Balearic Islands. Biol Conserv 122:611–619
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Vázquez DP, Aizen MA (2004) Asymetric specialization: a pervasive feature of plant-pollinator interactions. Ecology 85:1251–1257
Vázquez DP, Morris WF, Jordano P (2005) Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol Lett 8:1088–1094
Vázquez DP, Simberloff D (2003) Changes in interaction biodiversity induced by an introduced ungulate. Ecol Lett 6:1077–1083
Vázquez DP, Melián CJ, Wiliams NM, Blüthgen N, Krasnov BR, Poulin R (2007) Species abundance and asymmetric interaction strength in ecological networks. Oikos 116:1120–1127
Vilà M, D’Antonio CM (1998) Hybrid vigor for clonal growth in Carpobrotus (aizoaceae) in costal California. Ecol Appl 8:1196–1205
Vilà M, et al. (2006) Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33:853–861
Waser NM, Chittka L, Price MV, Williams NM, Ollerton J (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060
Weiss MR, Papaj DR (2003) Colour learning in two behavioural contexts: how much can a butterfly keep in mind? Anim Behav 65:425–434
Westrich P (1990) Die Wildbienen Baden-Württembergs. Ulmer, Stuttgart
Acknowledgements
We thank J. Bosch for insect identification supervision, L. Marco for the field assistance; and J. Bascompte, P. Jordano, A. Valido, K. Gross and three anonymous reviewers for comments on a previous version of the manuscript. Partial research support was provided by the Integrated European Project Assessing Large Scale Risks to Biodiversity with Tested Methods (ALARM: http://www.alarmproject.net), contract 506675 and the Ministerio de Ciencia y Tecnología projects Efecto de las Especies Invasoras en las Redes de Polinización (INVASRED) and Determinantes Biológicos del Riesgo de Invasiones Vegetales (RINVE). This work complies with the current laws of Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katherine Gross.
Rights and permissions
About this article
Cite this article
Bartomeus, I., Vilà, M. & Santamaría, L. Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 155, 761–770 (2008). https://doi.org/10.1007/s00442-007-0946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0946-1