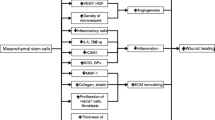
Abstract
The stem cells with their distinct ability of self-renewal and differentiation are considered an innovation in wound healing. However, there is lack of studies comparing the differential effect of the type and administration route of stem cells in the wound healing context. Thus, the current study has been designed to elucidate the effect of two of the most important stem cell types—the bone marrow-derived and adipose-derived stem cells in full thickness wound healing—and to evaluate, in this optimized wound model, the effectiveness of intradermal versus intravenous routes using H&E, Masson’s trichrome, and PKH26-stained sections. It also evaluated the immunohistochemical expression of the stem cell-related surface markers—Ki67, CD71, CD146, CD90, and CD163—and also assessed the level of TNFα and gene expression of NF-κB as two important inflammatory markers. The study revealed that the adipose stem cell groups have shown statistically significant improvement in inflammation, granulation tissue re-organization, and collagen deposition relative to their bone marrow-treated counterparts. The intradermally treated adipose stem cell group, in particular, has demonstrated the most supreme features regarding the expression of the proliferation-related surface markers Ki67 and CD71 as well as in the expression of CD90 in keratinocytes and hair follicle dermal sheaths. The same group has shown the lowest level of TNFα and the best outcome in the parameters of neo-epidermal thickness, granulation tissue re-organization, and pattern of collagen deposition. The systemically treated wounds have displayed superior expression of CD146-positive endothelial cells and dermal fibroblasts as well as better expression of CD163+ macrophages.








Similar content being viewed by others
References
Anfosso F, Bardin N, Vivier E, Sabatier F, Sampol J, Dignat-George F (2001) Outside-in signaling pathway linked to CD146 engagement in human endothelial cells. J Biol Chem 276(2):1564–1569
Ansell DM, Izeta A (2015) Pericytes in wound healing: friend or foe? Exp Dermatol 24(11):833–834
Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Kishimoto S (2006) Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell–mediated microvascular remodeling. Circulation 113(20):2413–2424
Bardin N, Anfosso F, Massé JM, Cramer E, Sabatier F, Le Bivic A, Sampol J, Dignat-George F (2001) Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood 98(13):3677–3684
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601
Bayati V, Hashemitabar M, Gazor R, Nejatbakhsh R, Bijannejad D (2013) Expression of surface markers and myogenic potential of rat bone marrow-and adipose-derived stem cells: a comparative study. Anatomy & cell biology 46(2):113–121
Betz P, Nerlich A, Wilske J, Tübel J, Penning R, Eisenmengen W (1993) The time-dependent localization of Ki 67 antigen-positive cells in human skin wounds. Int J Legal Med 106(1):35–40
Blocki A, Wang Y, Koch M, Peh P, Beyer S, Law P, Hui J, Raghunath M (2013) Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev 22(17):2347–2355
Boyle JJ (2012) Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr Opin Lipidol 23(5):453–461
Cammarota F, Laukkanen MO (2015) Mesenchymal stem/stromal cells in stromal evolution and cancer progression. Stem Cells Int 2016:4824573
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084
Carrion FA, Figueroa FE (2011) Mesenchymal stem cells for the treatment of systemic lupus erythematosus: is the cure for connective tissue diseases within connective tissue? Stem Cell Res Ther 2(3):23
Carvajal-Gonzalez JM, Roman AC, Cerezo-Guisado MI, Rico-Leo EM, Martin-Partido G, Fernandez-Salguero PM (2009) Loss of dioxin-receptor expression accelerates wound healing in vivo by a mechanism involving TGFβ. J Cell Sci 122(11):1823–1833
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011
Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4):e1886
Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC (2010) Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE 2-dependent mechanism. Clin Immunol 135(3):448–458
Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ (2011) Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 118(2):330–338
Chung E, Son Y (2014) Crosstalk between mesenchymal stem cells and macrophages in tissue repair. Tissue Eng Regen Med 11(6):431–438
Dong HY, Wilkes S, Yang H (2011) CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: a comparative immunochemical study with glycophorin A and hemoglobin A. Am J Surg Pathol 35(5):723–732
Du YY, Zhou SH, Zhou T, Su H, Pan HW, Du WH, Liu B, Liu QM (2008) Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy 10:469–478
Eggenhofer E, Hoogduijn MJ (2012) Mesenchymal stem cell-educated macrophages. Transplant Res 1(1):12
Evans BJ, Haskard DO, Sempowksi G, Landis RC (2013) Evolution of the macrophage CD163 phenotype and cytokine profiles in a human model of resolving inflammation. Int J Inflamm 2013
Ferreira D, Alvarado A, Moron CU, Romero-Sandoval E (2016) (275) interaction among keratinocytes, fibroblasts and CD163-overexpressing macrophages for a more efficient wound healing process. J Pain 17(4):S44–S45
Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V (2011) Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146(5):761–771
Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr (2009) Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18(5):683–692
Folgiero V, Migliano E, Tedesco M, Iacovelli S, Bon G, Torre ML, Sacchi A, Marazzi M, Bucher S, Falcioni R (2010) Purification and characterization of adipose-derived stem cells from patients with lipoaspirate transplant. Cell Med 1(1):3–14
Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24(4):150–154
Gillitzer R, Goebeler M (2001) Chemokines in cutaneous wound healing. J Leukoc Biol 69(4):513–521
Giri P, Ebert S, Braumann UD, Kremer M, Giri S, Machens HG, Bader A (2015) Skin regeneration in deep second-degree scald injuries either by infusion pumping or topical application of recombinant human erythropoietin gel. Drug Des devel ther 9:2565
Gomathysankar S, Halim AS, Yaacob NS (2014) Proliferation of keratinocytes induced by adipose-derived stem cells on a chitosan scaffold and its role in wound healing, a review. Arch Plast Surg 41(5):452
Guo Z, Draheim K, Lyle S (2011) Isolation and culture of adult epithelial stem cells from human skin. J Vis Exp: JoVE (49)
Hassan WU, Greiser U, Wang W (2014) Role of adipose-derived stem cells in wound healing. Wound Repair Regen 22(3):313–325
Hattori H, Ishihara M (2015) Altered protein secretions during interactions between adipose tissue-or bone marrow-derived stromal cells and inflammatory cells. Stem Cell Res Ther 6:70
Hilmi M, Bakar A, Halim AS, Jaafar H, Asiah AB, Hassan A (2013) Chitosan dermal substitute and chitosan skin substitute contribute to accelerated full-thickness wound healing in irradiated rats. Biomed Res Int 2013
Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX, Lai TS, Cheung AT, Walmsley GG, Chung MT, McArdle A, Longaker MT (2014) Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng 42:1494–1507
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Kawaguchi K, Katsuyama Y, Kikkawa S, Setsu T, Terashima T (2010) PKH26 is an excellent retrograde and anterograde fluorescent tracer characterized by a small injection site and strong fluorescence emission. Arch Histol Cytol 73:65–72
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301
Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J (2006) Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med 203(5):1235–1247
Kim WS, Park BS, Sung JH, Yang JM, Park SB et al (2007) Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48(1):15–24
Kim WS, Park BS, Sung JH (2009) Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res 301(5):329–336
Kisselbach L, Merges M, Bossie A, Boyd A (2009) CD90 expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology 59(1):31–44
Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP (2001) Fibroblast heterogeneity: existence of functionally distinct Thy 1+ and Thy 1—human female reproductive tract fibroblasts. Am J Pathol 159(3):925–935
Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP (2003) Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol 163(4):1291–1300
Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA (2010) Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 28(12):2229–2238
Kuo YR, Wang CT, Cheng JT, Wang FS, Chiang YC, Wang CJ (2011) Bone marrow–derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast Reconstr Surg 128(4):872–880
Lau SK, Chu PG, Weiss LM (2004) CD163A specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol 122(5):794–801
Lavker RM, Sun TT, Oshima H, Barrandon Y, Akiyama M, Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D, Panteleyev AA (2003) Hair follicle stem cells. J Investig Dermatol Symp Proc 8:28–38
Lee EY, Xia Y, Kim WS, Kim MH, Kim TH et al (2009) Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17(4):540–547
Lee SH, Jin SY, Song JS, Seo KK, Cho KH (2012) Paracrine effects of adiposederived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 24(2):136–143
Letourneau PA, Menge TD, Wataha KA, Wade CE, Cox CS Jr, Holcomb JB, Pati S (2011) Human bone marrow derived mesenchymal stem cells regulate leukocyte-endothelial interactions and activation of transcription factor NF-kappa B. J Tissue Sci Eng:001
Li Y, Zheng L, Xu X, Song L, Li Y, Li W, Zhang S, Zhang F, Jin H (2013) Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res Ther 4:113
Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA (2001) Wound healing in MIP-1α−/− and MCP-1−/− mice. Am J Pathol 159(2):457–463
Maleki M, Ghanbarvand F, Behvarz MR, Ejtemaei M, Ghadirkhomi E (2014) Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells 7(2):118
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276(5309):75–81
Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA (2007) Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 170(4):1178–1191
McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, Bansal M, Li Y, Chopp M, Dulchavsky SA, Gautam SC (2006) Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen 14(4):471–478
Mercati F, Pascucci L, Ceccarelli P, Dall’Aglio C, Pedini V, Gargiulo AM (2009) Expression of mesenchymal stem cell marker CD90 on dermal sheath cells of the anagen hair follicle in canine species. Eur J Histochem: EJH 53(3)
Metral E, Damour O, Rachidi W (2014) Adipose-derived stem cells promotes skin homeostasis and prevents its senescence in in vitro skin model. J Investig Dermatol 134:S67–S67
Metral E, Bechetoille N, Demarne F, Rachidi W, Damour O (2017) α6 integrin (α6high)/transferrin receptor (CD71) low keratinocyte stem cells are more potent for generating reconstructed skin epidermis than rapid adherent cells. Int J Mol Sci 18(2):282
Moon KM, Park YH, Lee JS, Chae YB, Kim MM et al (2012) The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J Mol Sci 13(1):1239–1257
Nakamura Y, Muguruma Y, Yahata T, Miyatake H, Sakai D, Mochida J, Ando K (2006) Expression of CD90 on keratinocyte stem/progenitor cells. Br J Dermatol 154(6):1062–1070
Natesan S, Baer DG, Walters TJ, Babu M, Christy RJ (2010) Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng A 16(4):1369–1384
Nishimura Y, Ii M, Qin G, Hamada H, Asai J, Takenaka H, Kishimoto S (2012) CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Investig Dermatol 132(3):711–720
Nishino Y, Ebisawa K, Yamada Y, Okabe K, Kamei Y, Ueda M (2011) Human deciduous teeth dental pulp cells with basic fibroblast growth factor enhance wound healing of skin defect. J Craniofac Surg 22:438–442
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Vogel JC (2006) Characterization and isolation of stem cell–enriched human hair follicle bulge cells. J Clin Investig 116(1):249
Olszewski WL (2003) Stem cells of the human skin epithelium—can they be isolated and resume function as single-cells transplanted into recipient skin defects? Ann Transplant 9(4):34–36
Onuma H, Mastui C, Morohashi M (2001) Quantitative analysis of the proliferation of epidermal cells using a human skin organ culture system and the effect of DbcAMP using markers of proliferation (BrdU, Ki-67, PCNA). Arch Dermatol Res 293(3):133–138
Park BS, Jang KA, Sung JH, Park JS, Kwon YH et al (2008) Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 34(10):1323–1326
Pelizzo G, Avanzini MA, Cornaglia AI, Osti M, Romano P, Avolio L, Maccario R, Dominici M, De Silvestri A, Andreatta E, Costanzo F (2015) Mesenchymal stromal cells for cutaneous wound healing in a rabbit model: pre-clinical study applicable in the pediatric surgical setting. J Transl Med 13(1):219
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C (2008) Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 17(4):761–774
Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC (2004) Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis. Circ Res 94(1):119–126
Rafii S, Lyden D (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9(6):702–712
Raposio E, Guida C, Baldelli I, Curto M, Fiocca R, Kunkl A, Santi PL (2007) Characterization of multipotent cells from human adult hair follicles. Toxicol in Vitro 21(2):320–323
Raza K, Larsen T, Samaratunga N, Price AP, Meyer C, Matson A, Ehrhardt MJ, Fogas S, Tolar J, Hertz MI, Panoskaltsis-Mortari A (2014) MSC therapy attenuates obliterative bronchiolitis after murine bone marrow transplant. PLoS One 9:e109034
Redvers RP, Li A, Kaur P (2006) Side population in adult murine epidermis exhibits phenotypic and functional characteristics of keratinocyte stem cells. Proc Natl Acad Sci 103(35):13168–13173
Rege TA, Hagood JS (2006) Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 20(8):1045–1054
Rodriguez J, Boucher F, Lequeux C, Josset-Lamaugarny A, Rouyer O, Ardisson O, Rutschi H, Sigaudo-Roussel D, Damour O, Mojallal A (2015) Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther 6:241
Saalbach A, Haustein UF, Anderegg U (2000) A ligand of human thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy-1-positive microvascular endothelial cells and fibroblasts. J Investig Dermatol 115(5):882–888
Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K (2004) Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 40(6):1304–1311
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180(4):2581–2587
Schaer DJ, Alayash AI (2010) Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid Redox Signal 12(2):181–184
Schlüter H, Kaur P (2013) In vivo transplantation assay at limiting dilution to identify the intrinsic tissue reconstitutive capacity of keratinocyte stem cells and their progeny. Methods Mol Biol 989:165–182
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3):311–322
Schrage A, Loddenkemper C, Erben U, Lauer U, Hausdorf G, Jungblut PR, Klugewitz K (2008) Murine CD146 is widely expressed on endothelial cells and is recognized by the monoclonal antibody ME-9F1. Histochem Cell Biol 129(4):441–451
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Smith AN, Willis E, Chan VT, Muffley LA, Isik FF et al (2010) Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 316(1):48–54
Solovey AN, Gui L, Chang L, Enenstein J, Browne PV, Hebbel RP (2001) Identification and functional assessment of endothelial P1H12. J Lab Clin Med 138:322–331
Song SY, Jung JE, Jeon YR, Tark KC, Lew DH (2011) Determination of adipose-derived stem cell application on photo-aged fibroblasts, based on paracrine function. Cytotherapy 13(3):378–384
Stout RD (2010) Macrophage functional phenotypes: no alternatives in dermal wound healing? J Leukoc Biol 87(1):19–21
Thomas HM, Cowin AJ, Mills SJ (2017) The importance of Pericytes in healing: wounds and other pathologies. Int J Mol Sci 18(6)
Tian X, Brookes O, Battaglia G (2017) Pericytes from mesenchymal stem cells as a model for the blood-brain barrier. Sci Rep 7:39676
Tobita M, Orbay H, Mizuno H (2011) Adipose-derived stem cells: current findings and future perspectives. Discov Med 11(57):160–170
Vishnubalaji R, Manikandan M, Al-Nbaheen M, Kadalmani B, Aldahmash A, Alajez NM (2012) In vitro differentiation of human skin-derived multipotent stromal cells into putative endothelial-like cells. BMC Dev Biol 12(1):7
Wan J, Xia L, Liang W, Liu Y, Cai Q (2013) Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res 2013
Watt SM, Athanassopoulos A, Harris AL, Tsaknakis G (2010) Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface 7(Suppl 6):S731–S751
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870
Wetzel A, Chavakis T, Preissner KT, Sticherling M, Haustein UF, Anderegg U, Saalbach A (2004) Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Immunol 172(6):3850–3859
Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25:2648–2659
Wu Y, Huang S, Enhe J, Fu X (2012) Insights into bone marrow-derived mesenchymal stem cells safety for cutaneous repair and regeneration. Int Wound J 9:586–594
Yamaguchi Y, Kubo T, Murakami T, Takahashi M, Hakamata Y, Kobayashi E, Yoshida S, Hosokawa K, Yoshikawa K, Itami S (2005) Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wounds with exposed bones when combined with an occlusive dressing. Br J Dermatol 152(4):616–622
Yan X, Lin Y, Yang D, Shen Y, Yuan M, Zhang Z, Li P, Xia H, Li L, Luo D, Liu Q (2003) A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood 102(1):184–191
Yan X, Cen Y, Wang Q (2016) Mesenchymal stem cells alleviate experimental rheumatoid arthritis through microRNA-regulated IκB expression. Sci Rep 6
Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208(1):64–76
Zhu X, Shi W, Tai W, Liu F (2012) The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived mesenchymal stem cells. Cell Tissue Res 350(2):277–287
Author information
Authors and Affiliations
Ethics declarations
The experiment was conducted in accordance with the guidelines of the international committee of use of laboratory animals. The experimental protocol including the way of animal treatment, anesthesia before induction of the surgical skin wound and methodology of collecting the stem cells were all approved from The Research Ethical Committee of the Faculty of Medicine, Cairo University.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Fig. S1
(A) Phase contrast images of rat of BM-SC isolated after 14 days. The majority of cells were spindle-like or star-like and BM-SCs were around 80% confluent. Lifted cells after trypsinization were small and rounded. (B) Flow cytometry of most of cultured adherent cells was positive to CD 29 while, (C) most of the adherent cells were negative for CD34 by flow cytometry. (D) Phase contrast images of rat of ADSC. Confluent rat ADSCs isolated after 14 days revealing fibroblastic morphology in the form of spindle shaped cells. (E) ADSC positive for CD73, and (F) negative for CD34 by flow cytometric analysis. (JPG 2312 kb)
Fig. S2
Flow cytometric characterization of BMSCs and ADSCs surface markers. (JPG 113 kb)
Rights and permissions
About this article
Cite this article
Aboulhoda, B.E., Abd el Fattah, S. Bone marrow-derived versus adipose-derived stem cells in wound healing: value and route of administration. Cell Tissue Res 374, 285–302 (2018). https://doi.org/10.1007/s00441-018-2879-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2879-x