Abstract
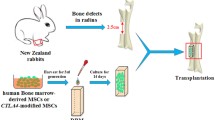
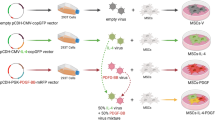
The improved ectopic osteogenesis of cytotoxic T-lymphocyte–associated antigen 4-Ig-modified bone marrow mesenchymal stem cells (MSCs-CTLA4) has been demonstrated but the mechanisms involved remain to be determined. The extracellular matrix (ECM) has recently been reported to play a vital role in bone formation and periostin (POSTN) has been suggested as a key member in constructing the ECM in bone tissue. We found that POSTN expression in the MSCs-CTLA4 group is significantly enhanced compared with that in the MSCs group, not only in tissue-engineered bone (TEB) with femur heterotopic transplantation in vivo but also under the immune activation condition in vitro. This ectopic osteogenesis effect is in accordance with POSTN expression. We also found that the soluble POSTN treatment up-regulates osteogenic marker expression in MSCs, including runt-related transcription factor 2, collagen 1, osteocalcin, osterix, and alkaline phosphatase and calcium nodule formation. These effects are diminished when the soluble POSTN is neutralized. Our results demonstrate that POSTN promotes the osteogenic differentiation of MSCs and that CTLA4 enhances the ectopic osteogenesis of MSCs-CTLA4-based TEB, potentially by maintaining POSTN expression in xenotransplantation.






Similar content being viewed by others
References
Alford AI, Kozloff KM, Hankenson KD (2015) Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol 65:20–31
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S (2013) Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PLoS One 8:e78347
Bonnet N, Garnero P, Ferrari S (2015) Periostin action in bone. Mol Cell Endocrinol 432:75–82
Boskey AL (1996) Matrix proteins and mineralization: an overview. Connect Tissue Res 35:357–363
Camacho NP, Landis WJ, Boskey AL (1996) Mineral changes in a mouse model of osteogenesis imperfecta detected by Fourier transform infrared microscopy. Connect Tissue Res 35:259–265
Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P (2006) Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 107:4817–4824
Dai F, Shi D, He W, Wu J, Luo G, Yi S, Xu J, Chen X (2006) hCTLA4-gene modified human bone marrow-derived mesenchymal stem cells as allogeneic seed cells in bone tissue engineering. Tissue Eng 12:2583–2590
Dai F, Yang S, Zhang F, Shi D, Zhang Z, Wu J, Xu J (2014) hTERT- and hCTLA4Ig-expressing human bone marrow-derived mesenchymal stem cells: in vitro and in vivo characterization and osteogenic differentiation. J Tissue Eng Regen Med 11:400–411
Dai F, Zhang F, Sun D, Zhang ZH, Dong SW, Xu JZ (2015) CTLA4 enhances the osteogenic differentiation of allogeneic human mesenchymal stem cells in a model of immune activation. Braz J Med Biol Res 48:629–636
Damsky CH (1999) Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 25:95–96
Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG (2005) Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 26:971–977
Engelhardt JJ, Sullivan TJ, Allison JP (2006) CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol 177:1052–1061
Ghatak S, Misra S, Norris RA, Moreno-Rodriguez RA, Hoffman S, Levine RA, Hascall VC, Markwald RR (2014) Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem 289:8545–8561
Gorski JP (2011) Biomineralization of bone: a fresh view of the roles of non-collagenous proteins. Front Biosci (Landmark Ed) 16:2598–2621
Hidalgo-Bastida LA, Cartmell SH (2010) Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng Part B Rev 16:405–412
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S (2011) Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med 17:1594–1601
Merle B, Garnero P (2012) The multiple facets of periostin in bone metabolism. Osteoporos Int 23:1199–1212
Morra L, Moch H (2011) Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch 459:465–475
Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101:695–711
Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ (2005) Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131–11144
Sa Q, Woodward J, Suzuki Y (2013) IL-2 produced by CD8(+) immune T cells can augment their IFN-gamma production independently from their proliferation in the secondary response to an intracellular pathogen. J Immunol 190:2199–2207
Sponer P, Kucera T, Diaz-Garcia D, Filip S (2014) The role of mesenchymal stem cells in bone repair and regeneration. Eur J Orthop Surg Traumatol 24:257–262
Tkatchenko TV, Moreno-Rodriguez RA, Conway SJ, Molkentin JD, Markwald RR, Tkatchenko AV (2009) Lack of periostin leads to suppression of Notch1 signaling and calcific aortic valve disease. Physiol Genomics 39:160–168
Yoshiba N, Yoshiba K, Hosoya A, Saito M, Yokoi T, Okiji T, Amizuka N, Ozawa H (2007) Association of TIMP-2 with extracellular matrix exposed to mechanical stress and its co-distribution with periostin during mouse mandible development. Cell Tissue Res 330:133–145
Young MF (2003) Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int 14:S35–S42
Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F (2015) EphB4 promotes osteogenesis of CTLA4-modified bone marrow-derived mesenchymal stem cells through cross talk with Wnt pathway in xenotransplantation. Tissue Eng Part A 21:2404–2416
Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F (2016) Periostin: a downstream mediator of EphB4-induced osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Stem Cells Int 2016:7241829
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K (2006) Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4:111–121
Zomorodian E, Baghaban Eslaminejad M (2012) Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int 2012:980353
Acknowledgements
The authors declare that they have no conflict of interest. This study was funded by the National Natural Science Foundation of China (grant number: 81601627, 31170931). The manuscript was edited and proofread by Medjaden Bioscience.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, F., Rong, Z., Wang, Z. et al. Periostin promotes ectopic osteogenesis of CTLA4-modified bone marrow mesenchymal stem cells. Cell Tissue Res 370, 143–151 (2017). https://doi.org/10.1007/s00441-017-2655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2655-3