Abstract
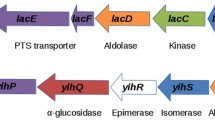
Enteric bacteria (Enteriobacteriaceae) carry on their single chromosome about 4000 genes that all strains have in common (referred to here as “obligatory genes”), and up to 1300 “facultative” genes that vary from strain to strain and from species to species. In closely related species, obligatory and facultative genes are orthologous genes that are found at similar loci. We have analyzed a set of facultative genes involved in the degradation of the carbohydrates galactitol, D-tagatose, D-galactosamine and N-acetyl-galactosamine in various pathogenic and non-pathogenic strains of these bacteria. The four carbohydrates are transported into the cell by phosphotransferase (PTS) uptake systems, and are metabolized by closely related or even identical catabolic enzymes via pathways that share several intermediates. In about 60% of Escherichia coli strains the genes for galactitol degradation map to a gat operon at 46.8 min. In strains of Salmonella enterica, Klebsiella pneumoniae and K. oxytoca, the corresponding gat genes, although orthologous to their E. coli counterparts, are found at 70.7 min, clustered in a regulon together with three tag genes for the degradation of D-tagatose, an isomer of D-fructose. In contrast, in all the E. coli strains tested, this chromosomal site was found to be occupied by an aga/kba gene cluster for the degradation of D-galactosamine and N-acetyl-galactosamine. The aga/kba and the tag genes were paralogous either to the gat cluster or to the fru genes for degradation of D-fructose. Finally, in more then 90% of strains of both Klebsiella species, and in about 5% of the E. coli strains, two operons were found at 46.8 min that comprise paralogous genes for catabolism of the isomers D-arabinitol (genes atl or dal) and ribitol (genes rtl or rbt). In these strains gat genes were invariably absent from this location, and they were totally absent in S. enterica. These results strongly indicate that these various gene clusters and metabolic pathways have been subject to convergent evolution among the Enterobacteriaceae. This apparently involved recent horizontal gene transfer and recombination events, as indicated by major chromosomal rearrangements found in their immediate vicinity.




Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) (1990) Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York
Bäumler AJ, Heffron F (1998) Mosaic structure of the smpB-nrdE intergenic region of Salmonella enterica. J Bacteriol 180:2220–2223
Berlyn MKB (1998) Linkage map of Escherichia coli K-12, Edition 10: the traditional map. Microbiol Mol Rev 62:814–894
Blattner FR, et al (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474
Bockmann J, Heuel H, Lengeler JW (1992) Characterization of a chromosomal non-PTS metabolic pathway for sucrose utilization in Escherichia coli EC3132. Mol Gen Genet 235:22–32
Bork P, Sander C, Valencia A (1993) Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase and galactokinase families of sugar kinases. Protein Sci 2:31–40
Boyd EF, Hartl DL (1998) Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J Bacteriol 180:1159–1165
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quanties of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brenner DJ (1984) Facultatively anaerobic gram-negative rods, family I Enterobacteriaceae. In: Krieg JN, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore-London, pp 408–516
Brinkkötter A, Klöss H, Alpert C-A, Lengeler JW (2000) Pathways for the utilization of N-acetyl-galactosamine and galactosamine in Escherichia coli. Mol Microbiol 37:125–135
Brinkkötter A, Shakeri-Garakani A, Lengeler JW (2002) New class II D-tagatose-bisphosphate aldolases from enteric bacteria. Arch Microbiol 177:410–419
Delidakis CE, Jones-Mortimer MC, Kornberg HL (1982) A mutant inducible for galactitol utilization in Escherichia coli. J Gen Microbiol 128:601–604
Demerec M, Fano U (1945) Bacteriophage-resistant mutants in Escherichia coli. Genetics 30:119–136
Dougan G, Haque A, Pickard D, Frankel G, O’Goara P, Wain J (2001) The Escherichia coli gene pool. Curr Opinion Microbiol 4:90–94
Eisen JA (2001) Gastrogenomics. Nature 409:463–466
Emmerth M, Goebel W, Miller SI, Hueck CJ (1999) Genomic subtraction identifies Salmonella typhimurium prophages, F-related plasmid sequences, and a novel fimbrial operon, stf , which are absent in Salmonella typhi. J Bacteriol 181:5652–5661
Farmer JJ III, Davis BR, Hickman-Brenner FW, McWhorter A, Huntley-Carter GP, Asbury MA, Riddle C, Wathen-Grady HG, Elias C, Fanning GR, Steigerwalt AG, O’Hara CM, Morris GK, Smith PB, Brenner DJ (1985) Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol 21:46–76
Groisman EA, Ochman H (2000) The path to Salmonella. Multiple horizontally-acquired genes are responsible for the specific virulence properties of Salmonella. ASM News 66:21–27
Grübl G, Vogler AP, Lengeler JW (1990) Involvement of the histidine protein (HPr) of the phosphotransferase system in chemotactic signaling of Escherichia coli K-12. J Bacteriol 172:5871–5876
Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H (1997) Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol 23:1089–1097
Hartley BS (1984) The structure and control of the pentitol operons. In: Mortlock RP (ed) Microorganisms as model systems for studying evolution. Plenum Press, New York, pp 55–107
Heuel H, Turgut S, Schmid K, Lengeler JW (1997) Substrate recognition domains as revealed by active hybrids between the D-arabinitol and ribitol transporters from Klebsiella pneumoniae. J Bacteriol 179:6014–6019
Heuel H, Shakeri-Garakani A, Turgut S, Lengeler JW (1998) Genes for D-arabinitol and ribitol catabolism from Klebsiella pneumoniae. Microbiology 144:1631–1639
Hochhut B, Jahreis K, Lengeler JW, Schmid K (1997) CTn scr94, a conjugative transposon found in enterobacteria. J Bacteriol 179:2097–2102
Hochhut B, Marrero J, Waldor MK (2000) Mobilization of plasmids and chromosomal DNA mediated by the SXT element, a constin found in Vibrio cholerae O139. J Bacteriol 182:2043–2047
Hurtado A, Rodriguez-Valera F (1999) Accessory DNA in the genomes of representatives of the Escherichia coli reference collection. J Bacteriol 181:2548–2554
Labedan B, Riley M (1999) Genetic inventory: Escherichia coli as a window on ancestral proteins. In: Charlebois RL (ed) Organization of the prokaryotic genome. ASM Press, Washington DC, pp 311–329
Lathe III WC, Snel B, Bork P (2000) Gene context conservation of a higher order than operons. Trends Biochem Sci 25:474–479
Lawrence JG, Roth JR (1999) Genomic flux: genome evolution by gene loss and acquisition. In: Charlebois RL (ed) Organization of the prokaryotic genome. ASM Press, Washington DC, pp. 263–289
Lengeler J (1977) Analysis of mutations affecting the dissimilation of galactitol (dulcitol) in Escherichia coli K12. Mol Gen Genet 152:83–91
Lengeler J, Lin ECC (1972) Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol 112:840–848
Lévy S, Zeng G-Q, Danchin A (1990) Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene 86:27–33
Link CD, Reiner AM (1983) Genotypic exclusion: a novel relationship between the ribitol-arabitol and galactitol genes of E. coli. Mol Gen Genet 189:337–339
Mahl MC, Wilson PW, Fife MA, Ewing HW (1965) Nitrogen fixation by members of the tribe Klebsielleae. J Bacteriol 89:1482–1487
McClelland M, Florea L, Sanderson K, Clifton SW, Parkhill J, Churcher C, Dougan G, Wilson RK, Miller W (2000) Comparison of the Escherichia coli K-12 genome with sampled genomes of a Klebsiella pneumoniae and three Salmonella enterica serovars, typhimurium, typhi and paratyphi. Nucleic Acids Res 28:4974–4986
Mobley HLT (2000) Virulence of the two primary uropathogens. Escherichia coli and Proteus mirabilis exhibit distinct mechanisms of pathogenesis when causing urinary tract infections. ASM News 66:403–410
Monterrubio R, Baldoma L, Obradors N, Aguilar J, Badia J (2000) A common regulator for the operons encoding the enzymes involved in D-galactarate, D-glucarate, and D-glycerate utilization in Escherichia coli. J Bacteriol 182:2672–2674
Mortlock RP (1984) The utilization of pentitols in studies of the evolution of enzyme pathways. In: Mortlock RP (ed) Microorganisms as model systems for studying evolution. Plenum Press, New York, pp 1–21
Nobelmann B, Lengeler JW (1995) Sequence of the gat operon for galactitol utilization from wild-type strain EC3132 of Escherichia coli. Biochim Biophys Acta 1262:69–72
Nobelmann B, Lengeler JW (1996) Molecular analysis of the gat genes for the galactitol transport and metabolism from a wild-type strain, EC3132, of Escherichia coli. J Bacteriol 178:6790–6795
Pellegrini M, Marcotte EM, Thompson MJ, Eisenberg D, Yeates TO (1999) Assigning protein functions by comparative genome analysis: protein phylogenetic profiles. Proc Natl Acad Sci USA 96:4285–4288
Pembroke JT, MacMahon C, McGrath B (2002) The role of conjugative transposons in the Enterobacteriaceae. Cell Mol Life Sci 2055–2064
Perna NT, et al (2001) Genome sequence of enterohaemorrhagic Escherichia coli 0157:H7. Nature 409:529–533
Podschun R (1990) Phenotypic properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zbl Hyg 189:527–535
Postma PW, Lengeler JW, Jacobson GR (1996) Phosphoenolpyruvate:carbohydrate phosphotransferase systems. In: Neidhardt FC, Curtiss III R, Ingraham JL, Lin ECCC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella (2nd edn), vol 1. ASM Press, Washington, DC, pp 1149–1174
Pupo GM, Lan R, Reeves PR (2000) Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci USA 97:10567–10572
Reiner AM (1975) Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol 123:530–536
Reiner AM (1977) Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol 132:166–173
Riley M, Anilionis A (1978) Evolution of the bacterial genome. Annu Rev Microbiol 32:519–560
Saier MH Jr (2000) Families of transmembrane sugar transport proteins. Mol Microbiol 35:699–710
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanderson KE, McClelland M, Liu S-L (1999) “Stable” genomes. In: Charlebois RL (ed) Organization of the prokaryotic genome. ASM Press, Washington DC, pp 217–233
Sanger F, Nicklen S, Coulsen AR (1977) DNA sequencing with chain-termination inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schaechter M and THE VIEW FROM HERE GROUP (2001) Escherichia coli and Salmonella 2000: the view from here. Microbiol Mol Biol Rev 65:119–130
Schmid K, Ebner R, Altenbuchner J, Schmitt R, Lengeler JW (1988) Plasmid-mediated sucrose metabolism in Escherichia coli K-12: mapping of the scr genes of pUR400. Mol Microbiology 2:1–8
Shakeri-Garakani A (1999) Mechanismen der stabilen Integration fremder Gene in das Chromosom der Enterobakterien am Beispiel der gat -, atl/rtl - und tag -Gene. PhD thesis, University of Osnabrück
Smith CL, Kloc SR, Cantor CR (1988) Pulsed-field gel electrophoresis and the technology of large DNA molecules. In: Davis KE (ed) Genome analysis: a practical approach. IRL Press, Oxford, pp 41–72
Sprenger GA, Lengeler JW (1987) Mapping of the sor -genes for L-sorbose degradation in the chromosome of Klebsiella pneumoniae. Mol Gen Genet 209:352–359
Thomas CM (ed) (2000) The horizontal gene pool. Bacterial plasmids and gene spread. Harwood Academic Publishers, Amsterdam
Wehmeier U, Sprenger GA, Lengeler JW (1989) The use of λplac Mu-hybrid phages in Klebsiella pneumoniae and the isolation of stable Hfr-strains. Mol Gen Genet 215:529–536
Wiman M, Bertani G, Kelly B, Sasaki I (1970) Genetic map of Escherichia coli strain C. Mol Gen Genet 107:1–31
Woodward MJ, Charles HP (1983) Polymorphism in Escherichia coli: rtl atl and gat regions behave as chromosomal alternatives. J Gen Microbiol 129:75–84
Acknowledgments
We would like to thank E. Placke and L. Schmieding for help in preparing the manuscript, the Deutsche Forschungsgemeinschaft for grants awarded to A. Shakeri-Garakani and A. Brinkkötter through the Graduiertenkolleg “Molekulare Zellbiologie/Membranbiologie”, and to J.W. Lengeler, K. Schmid, and S. Turgut through SFB171 and SFB 431, and the Verband der Chemischen Industrie for financial support to J.W. Lengeler.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Shakeri-Garakani, A., Brinkkötter, A., Schmid, K. et al. The genes and enzymes for the catabolism of galactitol, D-tagatose, and related carbohydrates in Klebsiella oxytoca M5a1 and other enteric bacteria display convergent evolution. Mol Genet Genomics 271, 717–728 (2004). https://doi.org/10.1007/s00438-004-1022-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-004-1022-8