Abstract
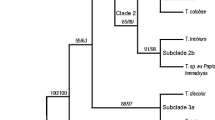
A molecular phylogenetic hypothesis is presented for the genus Trichuris based on sequence data from mitochondrial cytochrome c oxidase 1 (cox1) and cytochrome b (cob). The taxa consisted of nine populations of whipworm from five species of Sigmodontinae rodents from Argentina. Bayesian Inference, Maximum Parsimony, and Maximum Likelihood methods were used to infer phylogenies for each gene separately but also for the combined mitochondrial data and the combined mitochondrial and nuclear dataset. Phylogenetic results based on cox1 and cob mitochondrial DNA (mtDNA) revealed three clades strongly resolved corresponding to three different species (Trichuris navonae, Trichuris bainae, and Trichuris pardinasi) showing phylogeographic variation, but relationships among Trichuris species were poorly resolved. Phylogenetic reconstruction based on concatenated sequences had greater phylogenetic resolution for delimiting species and populations intra-specific of Trichuris than those based on partitioned genes. Thus, populations of T. bainae and T. pardinasi could be affected by geographical factors and co-divergence parasite-host.


Similar content being viewed by others
References
Anderson RC (2000) The Superfamily Trichinelloidea. In: Nematode parasites of vertebrates: their development and transmission, 2nth edn. CAB International, Wallingford, pp 605–621
Arrivillaga JC, Norris DE, Feliciangeli MD, Lanzaro GC (2002) Phylogeography of the neotropical sand fly Lutzomyia longipalpis inferred from mitochondrial DNA sequences. Infect Genet Evol 2:83–95
Babero BB, Cattan PE, Cabello C (1976) A new species of whipworm from the rodent Akodon longipilis in Chile. Trans Am Microsc Soc 95:232–235
Ballard JWO, Rand DM (2005) The population biology of mitochondrial DNA and its phylogenetic implications. Annu Rev Ecol Evol Syst 36:621–642
Betson M, Søe MJ, Nejsum P (2015) Human trichuriasis: whipworm genetics, phylogeny, transmission and future research directions. Curr Trop Med Rep 2:209–217
Bonvicino CR, Lindbergh SM, Maroja LS (2002) Small nonflying mammals from conserved and altered areas of Atlantic Forest and Cerrado: comments on their potential use for monitoring environment. Braz J Med Biol 62:1–12
Bundy DAP, Cooper ES (1989) Trichuris and trichuriasis in humans. Adv Parasitol 28:107–173
Bundy DAP, Kan SP, Rose R (1988) Age-related prevalence, intensity and frequency distribution of gastrointestinal helminth infection in urban slum children from Kuala Lumpur, Malaysia. Trans R Soc Trop Med Hyg 82:289–294
Cafrune MM, Aguirre DH, Rickard LG (1999) Recovery of Trichuris tenuis Chandler, 1930, from camelids (Lama glama and Vicugna vicugna) in Argentina. J Parasitol 85:961–962
Callejón R, De Rojas M, Nieberding C, Foronda P, Feliu C, Guevara D, Cutillas C (2010) Molecular evolution of Trichuris muris isolated from different Muridae hosts in Europe. Parasitol Res 107:631–641
Callejón R, De Rojas M, Feliu C, Balao F, Marrugal A, Henttonen H, Cutillas C (2012a) Phylogeography of Trichuris populations isolated from different Cricetidae rodents. Parasitology 139:1795–1812
Callejón R, Halajian A, De Rojas M, Marrugal A, Guevara DC, Cutillas C (2012b) 16S partial gene DNA and internal transcribes spacers ribosomal DNA as differential markers of Trichuris discolor populations. Vet Parasitol 186:350–363
Callejón R, Nadler S, De Rojas M, Zurita A, Petrášová J, Cutillas C (2013) Molecular characterization and phylogeny of whipworm nematodes inferred from DNA sequences of cox1 mtDNA and 18S rDNA. Parasitol Res 112:3933–3949
Callejón R, Cutillas C, Nadler SA (2015) Nuclear and mitochondrial genes for inferring Trichuris phylogeny. Parasitol Res 114:4591–4599
Cavallero S, De Liberato C, Friedrich KG, Di Cave D, Masella V, D’Amelio S, Berrilli F (2015) Genetic heterogeneity and phylogeny of Trichuris spp. from captive non-human primates based on ribosomal DNA sequence data. Infect Genet Evol 34:450–456
Cirignoli S, Teta P, Pardiñas UFJ, D’Elía G (2005) Oryzomyini Vorontsov 1959 (sensu Voss and Carleton 1993). In: Bárquez RM, Díaz MM, Ojeda RA (eds) Los Mamíferos de la Argentina. Sociedad Argentina para el Estudio de los Mamíferos, Argentina, pp 166–175
Cirignoli S, Galliari C, Pardiñas UFJ, Podestá D, Abramson R (2011) Mamíferos de la Reserva Valle del Cuña Pirú, Misiones, Argentina. Mastozool Neotrop 18:25–43
Correa-Gomes D, Lanfredi RM, Pinto RM, De Souza W (1992) Description of Trichuris travassosi n. sp. (Nematoda:Trichurinae) from a Brazilian rodent, by light and scanning electron microscopy. Mem Inst Oswaldo Cruz 87:1–10
Cox PG, Hautier L (2015) Evolution of the rodents. Advances in phylogeny, functional morphology, and development (eds) Cambridge University Press, 611 pp
Cubillos V, Torres P, Gallardo M (1991) Aspectos histopatológicos en un nuevo hospedador de Taenia taeniformis. Arch Med Vet 23:77–79
Cutillas C, German P, Arias P, Guevara DC (1996) Characterization of Trichuris skrjabini by isoenzyme gel electrophoresis: comparative study with Trichuris ovis. Acta Trop 62:63–69
Cutillas C, Oliveros R, De Rojas M, Guevara DC (2002) Determination of Trichuris muris from murid hosts and T. arvicolae (Nematoda) from arvicolid rodents by amplification and sequentiation of the ITS1-5.8S-ITS2 segment of the ribosomal DNA. Parasitol Res 88:574–582
Cutillas C, Oliveros R, De Rojas M, Guevara DC (2004) Determination of Trichuris skrjabini by sequentiation of the ITS1-5.8S-ITS2 segment of the ribosomal DNA. Comparative molecular study of different species of trichurids. J Parasitol 90:648–652
Cutillas C, De Rojas M, Ariza C, Úbeda JM, Guevara D (2007) Molecular identification of Trichuris vulpis and T. suis isolated from different hosts. Parasitol Res 100:383–389
Cutillas C, Callejón R, De Rojas M, Tewes B, Úbeda JM, Ariza C, Guevara DC (2009) Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop 11:299–307
Cutillas C, De Rojas M, Zurita A, Oliveros R, Callejón R (2014) Trichuris colobae n. sp. (Nematoda: Trichuridae), a new species of Trichuris from Colobus guereza kikuyensis. Parasitol Res 113:2725–2732
Cutillas C, De Rojas M, Zurita A, Oliveros R, Callejón R (2015) Trichuris colobae n. sp. (Nematoda: Trichuridae), a new species of Trichuris from Colobus guereza kikuyensis. Parasitol Res 113:2725–2732
D’Elia G, Pardiñas UFJ (2015) Subfamilia Sigmodontinae Wagner, 1843. In: Patton J, Pardiñas UFJ, D'Elia G (eds) Mammals of South America, 2nd edn. The University of Chicago Press, Rodents, pp 63–685
D’Elía G (2003) Rats, mice, and relatives IV: Sigmodontinae. In: Hutchins M, Geist V, Kleiman D, McDade M (eds) Grzimek’s animal life encyclopedia. Thomson-Gale, Farmington Hills, pp n263–n279
De la Sancha NU (2014) Patterns of small mammal diversity in fragments of subtropical Interior Atlantic Forest in eastern Paraguay. Mammalia. doi:10.1515/mammalia-2013-0100
Doležalová J, Oborník M, Hajdušková E, Jirků M, Petrželková KJ, Bolechová P, Cutillas C, Callejón R, Jaroš J, Beránková Z, Modrý D (2015) How many species of whipworms do we share? Whipworms from man and other primates form two phylogenetic lineages. Folia Parasitol 62:063. doi:10.14411/fp.2015.063
Feliú C, Spakulová M, Casanova JC, Renaud F, Morand S, Hugot JP, Santalla F, Durand P (2000) Genetic and morphological heterogeneity in small rodent whipworms in southwestern Europe: characterization of Trichuris muris and description of Trichuris arvicolae n. sp. (Nematoda: Trichuridae). J Parasitol 86:442–449
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Galliari C, Pardiñas UFJ, Goin F (1996) Lista comentada de los mamíferos argentinos. Mastozool Neotrop 3:39–61
Ghai RR, Simons ND, Chapman CA, Omeja PA, Davies TJ, Ting N, Goldberg TL (2014) Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Negl Trop Dis 8:e3256. doi:10.1371/journal.pntd.0003256
Grencis RK, Else KJ, Bancroft AJ, Bundy AP (1993) Trichuris Update ’93. Parasitol Today 9:309–310
Guardone L, Deplazes P, Macchioni F, Magi M, Mathis A (2013) Ribosomal and mitochondrial DNA analysis of Trichuridae nematodes of carnivores and small mammals. Vet Parasitol 197:364–36
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hawash MB, Andersen LO, Gasser RB, Stensvold CR, Nejsum P (2015) Mitochondrial genome analyses suggest multiple Trichuris species in humans, baboons, and pigs from different geographical regions. PLoS Negl Trop Dis 9:e0004059
Herrera HM, Norek A, Freitas TPT, Rademaker V, Fernandes O, Jansen AM (2005) Domestic and wild mammals infection by Trypanosoma evansi in a pristine area of the Brazilian Pantanal region. Parasitol Res 96:12–126
Jansen AM, Roque ALR (2010) Domestic and wild mammalian reservoirs. In: Telleria J, Tibayrenc M (eds) American trypanosomiasis: Chagas disease one hundred years of research. Elsevier, London, pp 249–276
Kramer KM, Monjeau JA, Birney EC, Sikes RS (1999) Phyllotis xanthopygus. Mamm Species 617:17
Kuhnen VV, Graipel ME, Pinto CJC (2012) Differences in richness and composition of gastrointestinal parasites of small rodents (Cricetidae, Rodentia) in a continental and insular area of the Atlantic forest in Santa Catarina state. Brazil Braz J Biol 72:563–567
Lallo MA, Pereira A, Araújo R, Favorito SE, Bertolla P, Bondan Fernandes E (2009) Ocorrência de Giardia, Cryptosporidium e microsporídios em animais silvestres em área de desmatamento no Estado de São Paulo, Brasil. Ciência Rural 39:1465–1470
Lanos-Cuentas EA, Roncal N, Villaseca P, Paz L, Ogusuku E, Perez JE (1999) Natural infections of Leishmania peruviana in animals in the Peruvian Andes. Trans R Soc Trop Med Hyg 93:15–20
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Liu GH, Gasser RB, Su A, Nejsum P, Peng L, Lin RQ, Li MW, Xu MJ, Zhu XQ (2012) Clear genetic distinctiveness between human- and pig-derived Trichuris based on analysis of mitochondrial datasets. PLoS Negl Trop Dis 6:e1539
Liu GH, Gasser RB, Nejsum P, Wang Y, Chen Q, Song HQ, Zhu XQ (2013) Mitochondrial and nuclear ribosomal DNA evidence supports the existence of a new Trichuris species in the endangered François’ leaf-monkey. PLoS ONE 8:e66249. doi:10.1371/journal.pone.0066249
Maldonado A, Simões R, Thiengo SC (2012) Angiostrongyliasis in the Americas. In: Lorenzo-Morales J (Eds.). Zoonosis Rijeka: In Tech 303–320
Mayle FE (2004) Assessment of the Neotropical dry forest refugia hypothesis in the light of palaeoecological data and vegetation model simulations. J Quat Sci 19:713–720
Miño MH, Rojas Herrera E, Notarnicola J (2013) The wild rodent Akodon azarae (Cricetidae: Sigmodontinae) as intermediate host of Taenia taeniaeformis (Cestoda: Cyclophyllidea) on poultry farms of central Argentina. Mastozool Neotrop 20:406–412
Musser GG, Carleton MD (2005) Superfamily Muroidea. In: Wilson DE, Reeder DA (eds) Mammal species of the world: a taxonomic and geographic reference. Johns Hopkins University Press, Baltimore, pp 894–1531
Nadler SA, Pérez-Ponce de León GPP (2011) Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology 138:1688–1709
Nagano I, Wu Z, Matsuo A, Pozio E, Takahashi Y (1999) Identification of Trichinella isolates by polymerase chain reaction-restriction fragment length polymorphism of the mitochondrial cytochrome c-oxidase subunit I gene. Int J Parasitol 29:1113–1120
Nissen S, Al-Jubury A, Hansen TV, Olsen A, Christensen H (2012) Genetic analysis of Trichuris suis and T. trichiura recovered from humans and pigs in a sympatric setting in Uganda. Vet Parasitol 188:68–77
Oliveros R, Cutillas C, De Rojas M, Arias P (2000) Characterization of four species of Trichuris (Nematoda: Enoplida) by their second internal transcribed spacer ribosomal DNA sequence. Parasitol Res 86:1008–1013
Pardiñas UFJ, Jayat JP (2008) Phyllotis bonariensis. The IUCN Red List of Threatened Species. Version 2014.2. <www.iucnredlist.org>. Downloaded on 29 July 2014
Pardiñas UF, Abba AM, Merino ML (2004) Micromamíferos (Didelphimorphia y Rodentia) del sudoeste de la provincia de Buenos Aires (Argentina): taxonomía y distribución. Mastozool Neotro 11:211–232
Pardiñas UF, D’Elia G, Cirignoli S, Suarez P (2005) A new species of Akodon (Rodentia, Cricetidae) from the Northern Campos grasslands of Argentina. J Mammal 86:462–474
Pardiñas UFJ, D’Elía G, Teta P, Ortiz PE, Jayat PJ, Cirignoli S (2006) Subfamilia Sigmodontini, Tribu Akodontini. In: Barquez RM, Díaz MM, Ojeda RA (eds) Mamíferos de Argentina, sistemática y distribución. Tucumán, Argentina, pp 146–202
Pardiñas UFJ, D’Elía G, Fagundes V, Christoff A, Geise L (2008) Akodon montensis. The IUCN Red List of Threatened Species. Version 2014.2. <www.iucnredlist.org>. Downloaded on 28 July 2014
Patton J, Catzeflis F, Weksler M, Percequillo A, D’Elia G, Pardiñas UFJ (2008) Thaptomys nigrita. The IUCN Red List of Threatened Species. Version 2014.2. <www.iucnredlist.org>. Downloaded on 31 July 2014
Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. J Biogeogr 27:261–273
Percequillo C, Weksler M, Pardiñas UFJ, D’Elía G, Teta P, Patterson B (2008) Sooretamys angouya. The IUCN Red List of Threatened Species. Version 2014.2. www.iucnredlist.org. Downloaded on 28 July 2014
Pérez-Ponce de León G, Nadler SA (2010) Critical Comment. What we don’t recognize can hurt us: a plea for awareness about cryptic species. J Parasitol 96:453–464
Polop J (1989) Distribution and ecological observations of wild rodents in Pampa de Achala, Córdoba, Argentina. Stud Neotrop Fauna Environ 24:53–59
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Ravasi DF, O’Riain MJ, Davids F, Illing N (2012) Phylogenetic evidence that two distinct Trichuris genotypes infect both humans and nonhuman primates. PLoS One 7:e44187
Reig OA (1987) An assessment of the systematics and evolution of the Akodontini with the description of new fossil species of Akodon (Cricetidae: Sigmodontinae). Fieldiana Zool 39:347–399
Reperant LA, Hegglin D, Tanner I, Fischer C, Deplazes P (2009) Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology 136:329–337
Ringuelet RA (1961) Rasgos fundamentales de la zoogeografia de la Argentina. Physis 22:151–170
Robles MR (2011) New species of Trichuris (Nematoda: Trichuridae) from Akodon montensis Thomas, 1913 of the Paranaense forest in Argentina. J Parasitol 97:319–327
Robles MR, Navone GT, Notarnicola J (2006) A new species of Trichuris (Nematoda: Trichuriidae) from Phyllotini rodents in Argentina. J Parasitol 92:100–104
Robles MR, Carballo MC, Navone GT (2008) A new species of Liniscus (Nematoda: Trichuridae) from Oxymycterus rufus and Akodon azarae (Cricetidae: Sigmodontinae) in Buenos Aires Province, Argentina. J Parasitol 94:909–917
Robles MR, Cutillas C, Panei CJ, Callejón R (2014) Morphological and molecular characterization of a new Trichuris species (Nematoda-Trichuridae), and phylogenetic relationships of Trichuris species of Cricetid rodents from Argentina. PLoS ONE 9:e112069. doi:10.1371/journal.pone.0112069
Robles MR, Kinsella JM, Galliari C, Navone GT (2016) New host, geographic records, and histopathologic studies of Angiostrongylus spp. (Nematoda: Angiostrongylidae) in rodents from Argentina, with updated summary of records from rodent hosts and host specificity assessment. Mem Inst Oswaldo Cruz 111:181–191
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Salaba O, Rylková K, Vadlejch J, Petrtýl M, Scháňková Š, Brožová A, Jankovská I, Jebavý L, Langrová I (2013) The first determination of Trichuris sp. from roe deer by amplification and sequentiation of the ITS1-5.8S-ITS2 segment of ribosomal DNA. Parasitol Res 112:955–960
Steppan SJ, Adkins RM, Anderson J (2004) Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol 53:533–553
Steppan SJ, Ramirez O, Banbury J, Huchon D, Pacheco V, Walker L, Spotorno AO (2007) A molecular reappraisal of the systematics of the leaf-eared mice Phyllotis and their relatives. In: Kelt DA, Lessa E, Salazar-Bravo J, Patton JL (eds) The quintessential naturalist: honoring the life and legacy of Oliver P. Pearson. University of California Press, Berkeley, pp 799–826
Subbotin SA, Vierstraete A, De Ley P, Rowe J, Waeyenberge L, Moens M, Vanfleteren JR (2001) Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Mol Phylogenet Evol 21:1–16
Suriano DM, Navone GT (1994) Three new species of the genus Trichuris Roederer 1761 (Nematoda-Trichuridae) from Caviomorph and Cricetid rodents in Argentina. Res Rev Parasitol 54:39–46
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Teta P, Pardiñas UFJ, Andrade A, Cirignoli S (2007) Distribución de los géneros Euryoryzomys y Sooretamys (Rodentia, Cricetidae) en Argentina. Mastozool Neotrop 14:279–284
Tiner JD (1950) Two new species of Trichuris from North America with description of Trichuris leporis (Nematoda: Aphasmidia). J Parasitol 36:350–354
Xie H, Bain O, Williams SA (1994) Molecular phylogenetic studies on filarial parasites based on 5S ribosomal spacer sequences. Parasite 1:141–151
Acknowledgments
We thank Carlos Galliari, Ulyses Pardiñas, Marcela Lareschi, Guillermo Panisse, Natalia Guerreiro Martins, and Juliana Notarnicola for their help during host collections; Carlos Galliari and Ulyses Pardiñas for the identification of the hosts; and Graciela T. Navone for her support in the laboratory. The present work was supported by a grant from the V Plan Propio de Investigación of the University of Sevilla, Spain, the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2010–0924), and Proyecto de incentivos de la Universidad Nacional de La Plata (UNLP N 627 and travel grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Rocío Callejón and María Del Rosario Robles contributed equally to this paper and should be considered as co-first authors
Rights and permissions
About this article
Cite this article
Callejón, R., Robles, M.D.R., Panei, C.J. et al. Molecular diversification of Trichuris spp. from Sigmodontinae (Cricetidae) rodents from Argentina based on mitochondrial DNA sequences. Parasitol Res 115, 2933–2945 (2016). https://doi.org/10.1007/s00436-016-5045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5045-y