Abstract
Purpose
The aim of this retrospective review was to compare the efficacy and safety of the atezolizumab plus carboplatin and nab-paclitaxel regimen versus the carboplatin and nab-paclitaxel regimen as front-line management for treatment-naïve, metastatic nonsquamous programmed death-ligand 1 (PD-L1)-positive non-small cell lung cancer (NSCLC) in a selected population.
Methods
Consecutive patients with untreated, metastatic nonsquamous PD-L1-positive NSCLC who initially received the atezolizumab plus carboplatin and nab-paclitaxel (ACN) regimen or carboplatin and nab-paclitaxel (CN) regimen were retrospectively identified in two medical institutions from 2017 to 2020. The co-primary end points were overall survival (OS) and progression-free survival (PFS); secondary end point was the rate of key adverse events (AEs).
Results
In sum, 171 patients were retrospectively analysed, 47 of whom were excluded according to the criteria used in this study, leaving 124 patients (ACN: n = 60, median age 64 years [range 46–75]; CN: n = 64, 63 years [47–72]). The median duration of follow-up was 27 months [range 1–37]. At the final follow-up, the median OS was 19.9 months (95% confidence interval [CI], 16.3–22.5) in the ACN group vs. 14.8 months (95% CI 12.5–17.2) in the CN group (hazard ratio [HR] 0.51, 95% CI 0.33–0.77; p = 0.001). A marked distinction in the median PFS was seen (8.5 months [95% CI 6.7–9.4] in the ACN group vs. in the CN group [5.1 months [95% CI 3.6–6.8; HR 0.60; 95% CI 0.38–0.95; p = 0.005]). The rates of the key AEs (neutropenia and anaemia) were greater in the ACN group than in the CN group (all p < 0.05), but these AEs were manageable.
Conclusion
Among selected populations of individuals with treatment-naïve, metastatic nonsquamous PD-L1-positive NSCLC, atezolizumab combined with carboplatin and nab-paclitaxel chemotherapy might have encouraging anticancer activity, with a tolerable safety profile.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 90% of patients, and that proportion is expected to continue to climb (Gettinger et al. 2016; Horn et al. 2017). Some patients tend to have an increased risk of metastatic nonsquamous NSCLC (Gettinger et al. 2015; Ready et al. 2019). Regardless of aetiology, in patients with metastatic nonsquamous NSCLC for whom available treatment options are lacking (Antonia et al. 2019; Horn et al. 2017), current front-line therapies for metastatic nonsquamous NSCLC are restricted by previous FDA-approved guidelines, and prognosis remains poor, with a median overall survival (OS) of about 10 months (Herbreteau et al. 2020; Mansfield et al. 2020). However, the landscape for metastatic nonsquamous NSCLC has been improving because ground-breaking, evidence-based treatments are increasingly accessible (Hida et al. 2018; Rittmeyer et al. 2017). Despite improvements in management, the 5-year survival rate for metastatic nonsquamous NSCLC varies from 2 to 30% (West et al. 2019). Differentiated management modalities for these patients seem to have become a crucial aspect of improving survival, but updates to metastatic nonsquamous NSCLC management concepts initiated by cancer immunotherapy research are still pending(Reck et al. 2020; West et al. 2019). So-called extended criteria with numerous immune checkpoint inhibitors have exhibited an encouraging clinical benefit of more than 1-year survival in patients with programmed cell death 1 (PD-1)-positive tumours (Reck et al. 2020), although currently, complementary approaches to metastatic nonsquamous NSCLC treatment are still in development (West et al. 2019).
Atezolizumab, an anti-PD-L1, humanised, engineered immunoglobulin G1 monoclonal antibody designed to block the binding of tumour cell-expressed programmed death-ligand 1 (PD-L1) to PD-1 and CD80 (B7.1), activates the immune response directed by T cells during tumour cell recognition and reverses the PD-L1/PD-1-mediated inhibition of T cells to reconstruct anticancer immunity by improving vascular endothelial growth factor (VEGF)-mediated immunomodulatory effects and chemotherapy-induced tumour cell antigen exposure (Furuya et al. 2021; Reck et al. 2020). Furthermore, combining atezolizumab with chemotherapy tend to have a synergistic effect in patients with NSCLC (Horn et al. 2018; Peters et al. 2017; Reck et al. 2020). The POPLAR trial(Fehrenbacher et al. 2016) and OAK trial (Rittmeyer et al. 2017) have indicated promising anticancer activity in pretreated metastatic nonsquamous PD-1-positive NSCLC, with median OS times of 12.6 months (95% confidence interval [CI] 9.7–16.4) and 13.8 months (11.8–15.7), respectively. However, to date, whether combining atezolizumab with cytotoxic chemotherapy can yield improved survival in individuals diagnosed with metastatic nonsquamous PD-1-positive NSCLC has remained unclear. Of note, the National Comprehensive Cancer Network (NCCN) management guiding principles have recommended immunotherapy-based combination regimens as front-line management for individuals with advanced NSCLC without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations (Ettinger et al. 2019). Unfortunately, published data on the effect of immunotherapy-based combination regimens in Chinese patients are lacking. Hence, we launched a multicentre retrospective study to compare the efficacy and safety of atezolizumab in combination with carboplatin and nab-paclitaxel regimen versus carboplatin and nab-paclitaxel regimen as front-line management for treatment-naïve stage IV nonsquamous PD-L1-positive NSCLC in selected populations of Chinese patients.
Materials and methods
Study design and patient eligibility
Between June 1, 2017, and June 30, 2020, consecutive individuals with treatment-naïve metastatic nonsquamous PD-L1-positive NSCLC who underwent front-line therapy with the atezolizumab plus carboplatin and nab-paclitaxel (ACN) regimen or carboplatin and nab-paclitaxel (CN) regimen in two medical centres (the First Affiliated Hospital, Sun Yat-sen University and the Tongji Medical College, Huazhong University of Science and Technology) and for whom patient characteristics were obtainable were retrospectively analysed. The following inclusion criteria were applied: clinically or histologically confirmed stage IV nonsquamous NSCLC; tumour cells expressing PD-L1 (tumour cells [TCs] or tumour-infiltrating immune cells [ICs] expressing PD-L1 ≥ 1%) (West et al. 2019); at least one cycle of front-line combined therapy; an Eastern Cooperative Oncology Group performance status score of 0 or 1; at least 1 calculable lesion per the modified response evaluation criteria in solid tumours (mRECIST); and adequate end-organ function (West et al. 2019). Major exclusion criteria included the following: a lack of patient characteristics; pretreated NSCLC (i.e. cytotoxic chemotherapy and/or radiotherapy, anti-PD-1 or anti-PD-L1 antibodies, or lung surgery); oncogenic driver mutations (i.e. EGFR or ALK mutations); discontinuity of front-line therapy, regardless of toxicity related to treatment; symptomatic central nervous system involvement; symptomatic deterioration attributed to disease progression per the mRECIST; contraindications listed in the drug leaflet; autoimmune-related diseases (i.e. systemic lupus erythaematosus, mandatory spondylitis, or autoimmune diabetes); bone marrow suppression; anaemia; and mental illness.
Study design and management
A retrospective multicentre review was executed in which eligible individuals had experienced the ACN or CN regimen for metastatic nonsquamous PD-L1-positive NSCLC. The decision to manage the disease by adopting the ACN or CN regimen was rendered by the chief thoracic surgeon (BX). Atezolizumab (1200 mg, intravenously) and carboplatin (area under the curve, 6 mg/mL/min) were administered intravenously every 3 weeks (West et al. 2019). Nab-paclitaxel was administered intravenously at 100 mg/m2 every week (West et al. 2019). The same drug delivery schedule was used in each case. Three weeks were considered a treatment cycle. Drug-related therapy was administered until the occurrence of unbearable toxicity, loss of clinical benefit, progression as per mRECIST, or death. Continuation of drug delivery after progression was permitted during the follow-up period. The concomitant therapies used were similar in the two regimens.
Outcomes and assessments
Tumour samples for PD-L1 testing were available from each patient. Immunohistochemistry analysis of PD-L1 expression on TCs and ICs was done with a PD-L1 IHC 28-8 pharmDx assay (Dako, Carpinteria, CA) as previously reported (Horn et al. 2017; Peters et al. 2017). TCs were categorised in accordance with pre-set values (1% ≤ TC1 < 5%, 5% ≤ TC1 < 50%, TC3 ≥ 50%) (West et al. 2019). PD-L1-positive NSCLC was defined as TC ≥ 1%. IC data were not required when recording baseline data. The co-primary end points were OS and progression-free survival (PFS). Tumour response was evaluated using contrast-enhanced CT unless contraindicated as per the mRECIST. OS and PFS were defined as the time from the beginning of front-line combined therapy to death from any cause and to progression or death from any cause, respectively. The secondary end point was the rate of major adverse events (AEs). AEs were graded as per National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Safety was evaluated as per major AEs reported during the entire treatment. Tumour response and progression were individually evaluated following the start of front-line combined therapy. Follow-up occurred every 3 weeks until insufferable toxicity or death, whichever occurred first.
Statistical analysis
The OS and PFS after the beginning of front-line combined therapy were assessed between the two treatment groups with a log-rank test. The estimation of median survival was executed using the Kaplan–Meier approach. The follow-up time was estimated with the reverse Kaplan–Meier method. The hazard ratios (HRs) and 95% CIs for median survival were assessed using a Cox regression model with a Cox proportional hazards model, with age, sex, ECOG-PS, time since diagnosis, PD-L1 tumour expression, tobacco use history, brain metastasis, number of metastatic sites utilised as covariates, and intervention was provided as the time-dependent variable. A two-tailed p value < 0.05 was regarded as significant. We analysed data using SPSS 26.0 software (IBM, Inc., New York).
Results
Demographic characteristics
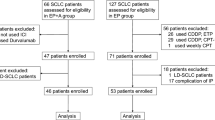
We identified 171 consecutive patients diagnosed with untreated metastatic nonsquamous PD-L1-positive NSCLC who had undergone the ACN or CN regimen, of whom 47 were excluded in accordance with the eligibility criteria. Thus, 124 eligible patients were evaluated. Among the 124 patients, 60 received the ACN regimen, and 64 received the CN regimen (Fig. 1). The patient characteristics are presented in Table 1. The median age was 64 years (range 46–75) in the ACN group and 63 years (47–72) in the CN group. In the ACN group, 68.3% of patients (n = 41) were male, 10.0% (n = 6) were never smokers, 60.0% (n = 36) had asymptomatic brain metastasis, and 78.3% (n = 47) had more than or equal to three metastatic sites. In the CN group, 52.8% of patients (n = 43) were male, 12.5% (n = 8) were never smokers, 57.8% (n = 37) had asymptomatic brain metastasis, and 75.0% (n = 48) had greater than or equal to three metastatic sites. ECOG-PS was 0 in 30.0% and 1 in 70.0% of individuals who underwent the ACN regimen versus 0 in 31.3% and 1 in 68.7% of patients who underwent the CN regimen (p = 0.881). PD-L1 tumour expression was TC1 in 25.0%, TC2 in 18.3%, and TC3 in 56.7% of patients who underwent the atezolizumab plus carboplatin and nab-paclitaxel regimen versus TC1 in 18.8%, TC2 in 21.9%, and TC3 in 59.3% of patients who underwent the carboplatin and nab-paclitaxel regimen (p = 0.708). The patient characteristics were well proportioned between the groups. At the final follow-up, 60 individuals in the ACN group and 64 in the CN group underwent at least one cycle of combination therapy. The median duration of combination therapy was similar between both cohorts (17 months [range 1–37] in the atezolizumab plus carboplatin and nab-paclitaxel regimen group and 16 months [1–36] in the carboplatin and nab-paclitaxel regimen group). No significant differences were detected in the number of administrations of nab-paclitaxel between both groups. The median cycles were 22 (range 1–28) in the ACN group and 21 (1–25) in the CN group.
Flow diagram displaying the methods adopted to identify patients to assess the efficacy and safety of the ACN regimen versus CN regimen as front-line management for metastatic nonsquamous PD-L1-positive NSCLC in selected population. ACN atezolizumab plus carboplatin and nab-paclitaxel; CN carboplatin and nab-paclitaxel; PD-L1 programmed cell death-ligand 1; NSCLC non-small cell lung cancer
Efficacy
The median follow-up time was similar between treatment groups (27 months [range 1–37] in the atezolizumab plus carboplatin and nab-paclitaxel regimen group and 27 months [1–36] in the carboplatin and nab-paclitaxel regimen group). The objective response rate of 53.3% (95% CI 45.1–57.8) in the ACN group was higher than the 29.7% (26.1–32.8) observed in the CN group (p = 0.008) (Table 2). For patients in the ACN group, the ORR was 11.7%, 18.3%, and 23.3% for cohorts TC1, TC2, and TC3, respectively. For patients in the CN group, the ORR was 3.1%, 10.9%, and 15.6% for cohorts TC1, TC2, and TC3, respectively. The ORR tend to be lower in patients with low PD-L1 expression compared with those with high PD-L1 expression. Significant differences were detected in reductions in tumour size per independent imaging review by mRECIST (65.5% [38 of 58] in the ACN group vs. 40.9% [25 of 61] in the CN group, p = 0.008), as presented in Figs. 2 and 3. The 3-, 12-, and 24-month OS rates were 98.3%, 91.4%, and 42.2%, respectively, in the ACN group and 96.8%, 71.8%, and 24.9%, respectively, in the CN group. A noticeable distinction was seen in the median OS between treatment cohorts (19.9 months [95% CI 16.3–22.5] in the ACN group vs. 14.8 months [12.5–17.2] in the CN group), as presented in Fig. 4. The ACN regimen drastically improved the median OS compared to the CN regimen and resulted in a 49% lower risk of death than the CN regimen (HR 0.51; 95% CI 0.33–0.77; p = 0.001). A noteworthy distinction of 5.1 months in the median OS was seen, and the superiority of the ACN regimen over the CN regimen may be positive because a separation of survival curves was seen throughout the follow-up period. In addition, a noteworthy distinction in the median PFS was noted (8.5 months [95% CI 6.7–9.4] in the ACN group vs. 5.1 months [3.6–6.8] in the CN regimen group [HR 0.60; 95% CI 0.38–0.95; p = 0.005]), as exhibited in Fig. 5.
Kaplan–Meier curves for OS. The median OS was 19.9 months (95% CI 16.3–22.5) for ACN and 14.8 months (95% CI 12.5–17.2) for CN (HR 0.51, 95% CI 0.33–0.77; p = 0.001). *HR was estimated with a Cox proportional hazards model, with the age, sex, ECOG-PS, time since diagnosis, PD-L1 tumour expression, tobacco use history, brain metastasis, number of metastatic sites utilised as covariates, and intervention provided as the time-dependent variable. OS overall survival; ACN atezolizumab plus carboplatin and nab-paclitaxel; CN carboplatin and nab-paclitaxel; HR hazard ratio; CI confidence interval; ECOG-PS Eastern Collaborative Oncology Group performance status; PD-L1 programmed cell death-ligand 1
Kaplan–Meier curves for PFS. The median PFS was 8.5 months (95% CI 6.7–9.4) for ACN and 5.1 months (95% CI 3.6–6.8) for CN (HR 0.60, 95% CI 0.38–0.95; p = 0.005). *HR was estimated with a Cox proportional hazards model, with the age, sex, ECOG-PS, time since diagnosis, PD-L1 tumour expression, tobacco use history, brain metastasis, number of metastatic sites utilised as covariates, and intervention provided as the time-dependent variable. PFS progression-free survival; ACN atezolizumab plus carboplatin and nab-paclitaxel; CN carboplatin and nab-paclitaxel; HR hazard ratio; CI confidence interval; ECOG-PS Eastern Collaborative Oncology Group performance status; PD-L1 programmed cell death-ligand 1
Subgroup analyses demonstrated that for individuals with TC3 PD-L1-expressing cells, the median OS was 23.7 months in the ACN group versus 16.1 months in the CN group (HR 0.25; p = 0.001); for individuals with TC2 PD-L1-expressing cells, the median OS was 19.1 months in the ACN group versus 15.3 months in the CN group (HR 0.39; p = 0.003); for individuals with TC1 PD-L1-expressing cells, the median OS was 16.8 months in the ACN group versus 12.6 months in the CN group (HR 0.12 months, p = 0.002).
Safety
In total, 106 patients (85.5%) had one or more AEs related to first-line therapy. Of the 124 patients, interruption related to AEs occurred in 13 (10.4%) patients (6 of 60 individuals in the ACN group vs. 7 of 64 individuals in the CN group, p = 0.865). Key treatment-related grade ≥ 3 AEs are shown in Table 3. The safety profile of ACN was in accordance with the earlier described safety profile, without unfamiliar AEs detected. The key grade 3 or worse AEs related to first-line therapy were neutropenia (19 [31.7%] of 60 in the ACN group vs. 6 [9.4%] of 64 in the CN group) and anaemia (15 [25.0%] in the ACN group vs. 7 [10.9%] in the CN group). There were no noteworthy distinctions in regard to other grade ≥ 3 AEs. Deaths related to first-line therapy were not observed in the study.
Discussion
The findings of this study demonstrate that the atezolizumab plus carboplatin and nab-paclitaxel regimen as front-line management might provide greater survival benefits in PD-L1-positive metastatic nonsquamous NSCLC patients in a selected population than the carboplatin and nab-paclitaxel regimen, with a tolerable safety profile. The high OS indicates that the treatment regimen incorporating atezolizumab with carboplatin and nab-paclitaxel might have synergistic effects and prevent the progression of tumours.
The study findings are consistent with the findings of a randomised, phase 3 trial (IMpower130) (West et al. 2019) that compared the clinical outcomes of atezolizumab plus carboplatin and nab-paclitaxel vs. carboplatin and nab-paclitaxel as front-line therapies in 723 individuals with pretreated advanced nonsquamous NSCLC. The results indicated that noteworthy improvements were detected in regard to median OS (18.6 months [95% CI 16.0–22.2] in the ACN group and 13.9 months [12.0–18.7] in the CN group) (HR 0.79; 95% CI 0.64–0.98; p = 0.033) and median PFS (7.0 months [95% CI 6.2–7.3] in the atezolizumab plus carboplatin and nab-paclitaxel regimen group and 5.5 months [4.4–5.9] in the carboplatin and nab-paclitaxel regimen group (HR 0.64; 95% CI 0.54–0.77; p < 0.0001)). This IMpower130 trial showed that the addition of atezolizumab to carboplatin and nab-paclitaxel is a promising anticancer option. Moreover, a trend towards excellent survival related to atezolizumab was noticed, irrespective of EGFR or ALK mutations. Similarly, a subgroup analysis of the phase 3 OAK trial (NCT02008227) (Hida et al. 2018) of 64 Japanese patients diagnosed with locally advanced PD-L1-positive NSCLC who underwent therapy with atezolizumab showed that a longer median OS was detected for the atezolizumab regimen than for the docetaxel regimen (21.3 months vs. 17.0 months, respectively; HR 0.80; 95% CI 0.41–1.57). In 2017, a phase 2 trial (Peters et al. 2017) of atezolizumab as a front-line or subsequent therapy for individuals with PD-L1-selected metastatic NSCLC (BIRCH) demonstrated that the median OS in an updated survival analysis of TC3 patients administered single-agent atezolizumab was 26.9 months; the median OS for patients administered atezolizumab combined with one prior platinum chemotherapy was 15.5 months; and the median OS for patients administered atezolizumab plus at least two prior chemotherapies was 13.2 months. In the BIRCH trial, prior cytotoxic chemotherapy did not appear to promote survival, and noteworthy OS improvement was detected in individuals with high TC3 PD-L1 expression.
Expression of PD-L1 in the tumour microenvironment tends to result in a poor prognosis (Borghaei et al. 2021; Gettinger et al. 2018; Gettinger et al. 2015; Haratani et al. 2018; Kazandjian et al. 2016). A high mutation rate related to NSCLC suggests that NSCLC cells may have a promising durability of response to immune checkpoint inhibitors (Borghaei et al. 2015; Gettinger et al. 2016; Owonikoko et al. 2021), although an association is lacking between tumour response and PD-L1 expression in the phase 1 trial of atezolizumab (Hellmann et al. 2020). Restoring tumour-specific T cell immunity using directly inhibiting PD-L1-PD-1 signalling has shown sustained anticancer activity in the management of PD-L1-positive NSCLC (Borghaei et al. 2021; Horn et al. 2018; Ikeda et al. 2021). Combining atezolizumab with carboplatin and nab-paclitaxel may have synergistic antitumour effects related to immune microenvironment modulation and might reduce or avoid the occurrence of immune escape and improve anticancer effects (Reck et al. 2020; West et al. 2019). An open-label phase IB study(Liu et al. 2018) of 26 patients with untreated stage IIIB/IV NSCLC who were administered atezolizumab plus carboplatin and nab-paclitaxel for four to six cycles, followed by atezolizumab maintenance, showed that the incorporation of atezolizumab into the carboplatin and nab-paclitaxel regimen was promising with a response rate of 46%, median OS of 17.0 months (95% CI 12.7–not evaluable), and median PFS of 5.7 months (95% CI 4.4–14.8).
Although individuals with PD-L1-positive NSCLC have a greater OS benefit attributed to atezolizumab, PD-L1 expression alone seems to partially explicate the OS benefit in individuals with PD-L1-positive NSCLC receiving atezolizumab plus cytotoxic chemotherapy for front-line management (Chalabi et al. 2020; Mansfield et al. 2020). Although NSCLC initially reacts strongly to cytotoxic chemotherapy, characterised by a substantial reduction in tumour burden, NSCLC often recurs or becomes resistant to chemotherapeutic drugs during or after treatment (Hopkins et al. 2021; von Pawel et al. 2019). The combination of VEGF-mediated immunomodulatory effects and chemotherapy-induced tumour cell antigen exposure may have a good synergistic anticancer effect (Ettinger et al. 2019; Reck et al. 2020). Stimulation of VEGF-mediated immunoregulation might be initiated by EGFR/ALK signalling (Peters et al. 2017). The enhancement of the anticancer immune response may be strongly associated with cytotoxic chemotherapy-induced exposure of tumour antigens (Reck et al. 2020). Although the IMpower130 trial (West et al. 2019) demonstrated that atezolizumab combined with carboplatin and nab-paclitaxel had a better OS benefit than carboplatin and nab-paclitaxel in front-line management of treatment-naïve, nonsquamous NSCLC in the wild-type population, the trial eliminated individuals with EGFR/ALK mutations and/or liver metastases and offer negligible survival benefit to these cases. A previous IMpower150 trial (Hopkins et al. 2021) compensated for these shortcomings, and the results from the trial further confirmed that the combination has anticancer activity in nonsquamous NSCLC.
The present study has several drawbacks. First, the retrospective design is associated with inherent biases. In the case of uncontrolled confounding, we cannot be sure of the magnitude of the effects we observed. Data with a high probability of misuse may be unavoidable and may have affected our results. Furthermore, regarding confusion issues, although we adopted strict inclusion criteria and checked all patients for potential diseases, these were at best only partial measures, and confusion issues may still have affected the analysis results. Second, during the follow-up period, the aetiology analysis of some deaths was not detailed. When difficulties in interpretation occurred, our diagnosis was cancer-related death, which may lead to uncertainty in the outcome because some patients may have died due to suicide or accident. In addition, when the follow-up data for individual patients were unclear or unavailable, indirect acquisition was used, which may have had an impact on the results. Third, the collection of patient baseline data was limited to the content recorded in the electronic health record. Errors may lie in patient memories and clinician records. Fourth, the generalizability of this study was limited to selected populations of Chinese patients diagnosed with treatment-naïve stage IV nonsquamous PD-L1-positive NSCLC.
Conclusion
The results from this review support the extending body of evidence indicating that combining atezolizumab with carboplatin and nab-paclitaxel chemotherapy might have encouraging survival benefits in patients with treatment-naïve metastatic nonsquamous PD-L1-positive NSCLC, with a tolerable safety profile. Given the retrospective nature of this study, we cannot draw any definite conclusions.
Data availability
All data generated or analysed during this study are included in the article.
Abbreviations
- PD-L1:
-
Programmed death-ligand 1
- PD-1:
-
Programmed cell death 1
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- AEs:
-
Adverse events
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- VEGF:
-
Vascular endothelial growth factor
- EGFR:
-
Epidermal growth factor receptor
- ALK:
-
Anaplastic lymphoma kinase
- TCs:
-
Tumour cells
- ICs:
-
Immune cells
- mRECIST:
-
Modified response evaluation criteria in solid tumours
References
Antonia SJ et al (2019) Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 20:1395–1408. https://doi.org/10.1016/s1470-2045(19)30407-3
Borghaei H et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Borghaei H et al (2021) Five-year outcomes from the randomized, phase iii trials checkMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 39:723–733. https://doi.org/10.1200/jco.20.01605
Chalabi M et al (2020) Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 31:525–531. https://doi.org/10.1016/j.annoc.2020.01.006
Ettinger DS et al (2019) NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 17:1464–1472. https://doi.org/10.6004/jnccn.2019.0059
Fehrenbacher L et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837–1846. https://doi.org/10.1016/s0140-6736(16)00587-0
Furuya N et al (2021) Real-world efficacy of atezolizumab in non-small cell lung cancer: a multicenter cohort study focused on performance status and retreatment after failure of anti-PD-1 antibody. Thoracic Cancer 12:613–618. https://doi.org/10.1111/1759-7714.13824
Gettinger SN et al (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33:2004–2012. https://doi.org/10.1200/jco.2014.58.3708
Gettinger S et al (2016) Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34:2980–2987. https://doi.org/10.1200/jco.2016.66.9929
Gettinger S et al (2018) Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol 36:1675–1684. https://doi.org/10.1200/jco.2017.77.0412
Haratani K et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. JAMA Oncol 4:374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Hellmann MD et al (2020) Phase 1 study of epacadostat in combination with atezolizumab for patients with previously treated advanced nonsmall cell lung cancer. Int J Cancer 147:1963–1969. https://doi.org/10.1002/ijc.32951
Herbreteau G et al (2020) Circulating tumor DNA as a prognostic determinant in small cell lung cancer patients receiving atezolizumab. J Clin Med 9:3861. https://doi.org/10.3390/jcm9123861
Hida T et al (2018) Atezolizumab in Japanese patients with previously treated advanced non-small-cell lung cancer: a subgroup analysis of the phase 3 OAK study. Clin Lung Cancer 19:E405–E415. https://doi.org/10.1016/j.cllc.2018.01.004
Hopkins AM, Kichenadasse G, Abuhelwa AY, McKinnon RA, Rowland A, Sorich MJ (2021) Value of the lung immune prognostic index in patients with non-small cell lung cancer initiating first-line atezolizumab combination therapy: subgroup analysis of the IMPOWER150 trial. Cancers (BAsel) 13:1176. https://doi.org/10.3390/cancers13051176
Horn L et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220–2229. https://doi.org/10.1056/NEJMoa1809064
Horn L et al (2017) Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35:3924–3933. https://doi.org/10.1200/jco.2017.74.3062
Ikeda M et al (2021) Longitudinal evaluation of PD-L1 expression on circulating tumor cells in non-small cell lung cancer patients treated with nivolumab. Cancers (basel) 13:2290. https://doi.org/10.3390/cancers13102290
Kazandjian D et al (2016) Benefit-risk summary of nivolumab for patients with metastatic squamous cell lung cancer after platinum-based chemotherapy: a report from the US Food and Drug Administration. JAMA Oncol 2:118–122. https://doi.org/10.1001/jamaoncol.2015.3934
Liu SV et al (2018) Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. Eur J Cancer 101:114–122. https://doi.org/10.1016/j.ejca.2018.06.033
Mansfield AS et al (2020) Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol 31:310–317. https://doi.org/10.1016/j.annonc.2019.10.021
Owonikoko TK et al (2021) Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: checkMate 451. J Clin Oncol 39:1349–1359. https://doi.org/10.1200/jco.20.02212
Peters S et al (2017) Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol 35:2781–2789. https://doi.org/10.1200/jco.2016.71.9476
Ready N et al (2019) First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol 37:992–1000. https://doi.org/10.1200/jco.18.01042
Reck M et al (2020) Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev Respir Med 14:125–136. https://doi.org/10.1080/17476348.2020.1701439
Rittmeyer A et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265. https://doi.org/10.1016/s0140-6736(16)32517-x
von Pawel J et al (2019) Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer 107:124–132. https://doi.org/10.1016/j.ejca.2018.11.020
West H et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924–937. https://doi.org/10.1016/s1470-2045(19)30167-6
Funding
This work failed to receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
BX, HC, and YL designed the study and prepared the manuscript. YL, XZ, and WY contributed to data collection. TL, WY, and WG contributed to study design, data collection, data analysis, and manuscript editing. All the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest associated with this manuscript.
Ethics approval
All the procedures implemented in studies involving human participants were consistent with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the Institutional Review Boards of the First Affiliated Hospital, Sun Yat-sen University (No. 15/3301) and the Tongji Medical College, Huazhong University of Science and Technology (No. SY4202).
Consent to participate
All the participants provided written informed consent and studies were approved by the local ethics committee.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, B., Cheng, H., Li, K. et al. Carboplatin and nab-paclitaxel chemotherapy with or without atezolizumab as front-line management for treatment-naïve metastatic nonsquamous non-small cell lung cancer with PD-L1 staining: a retrospective study. J Cancer Res Clin Oncol 148, 3029–3038 (2022). https://doi.org/10.1007/s00432-021-03873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03873-3