Abstract
Introduction
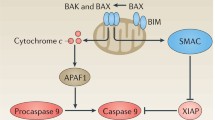
BCL-2 is the founding member of the BCL-2 family of apoptosis regulatory proteins that either induce (pro-apoptotic) or inhibit (anti-apoptotic) apoptosis. The anti-apoptotic BCL-2 is classified as an oncogene, as damage to the BCL-2 gene has been shown to cause a number of cancers, including lymphoma. Ongoing research has demonstrated that disruption of BCL-2 leads to cell death. BCL-2 is also known to be involved in the development of resistance to chemotherapeutic agents, further underscoring the importance of targeting the BCL-2 gene in cancer therapeutics. Thus, numerous approaches have been developed to block or modulate the production of BCL-2 at the RNA level using antisense oligonucleotides or at the protein level with BCL-2 inhibitors, such as the novel ABT737.
Methods
In this article, we briefly review previous strategies to target the BCL-2 gene and focus on a new approach to silence DNA, DNA interference (DNAi).
Results and conclusion
DNA interference is aimed at blocking BCL-2 gene transcription. Evaluations of this technology in preclinical and early clinical studies are very encouraging and strongly support further development of DNAi as cancer therapeutics. A pilot phase II clinical trial in patients with relapsed or refractory non-Hodgkin lymphoma, PNT2258 demonstrated clinical benefit in 11 of 13 patients with notable responses in diffuse large B cell lymphoma and follicular lymphoma. By targeting the DNA directly, the DNAi technology promises to be more effective compared with other gene-interference strategies that target the RNA or protein but leaves the dysregulated DNA functional.



Similar content being viewed by others
References
Abdullah LN, Chow EK-H (2013) Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med 2(1):3
Ackler S, Xiao Y, Mitten MJ, Foster K, Oleksijew A, Refici M, Bauch J (2008) ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther 7(10):3265–3274
Agrawal N, Dasaradhi P, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK (2003) RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev 67(4):657–685
Al-Katib AM, Sun Y, Goustin AS, Azmi AS, Chen B, Aboukameel A, Mohammad RM (2009). SMI of Bcl-2 TW-37 is active across a spectrum of B-cell tumors irrespective of their proliferative and differentiation status. J Hematol Oncol 2(8)
Arnold AA, Aboukameel A, Chen J, Yang D, Wang S, Al-Katib A, Mohammad RM (2008) Preclinical studies of Apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-X. Mol Cancer 7:20
Banerjee A, Qian P, Wu Z-S, Ren X, Steiner M, Bougen NM, Lobie PE (2012) Artemin stimulates radio- and chemo-resistance by promoting TWIST1-BCL-2-dependent cancer stem cell-like behavior in mammary carcinoma cells. J Biol Chem 287(51):42502–42515
Bennett CF, Swayze EE (2010) RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 50:259–293
Castanotto D, Stein CA (2014) Antisense oligonucleotides in cancer. Curr Opin Oncol 26(6):584–589
Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18(4):157–164
Cleary ML, Smith SD, Sklar J (1986) Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14; 18) translocation. Cell 47(1):19–28
Congmin G, Mu Z, Yihui M, Hanliang L (2006) Survivin-an attractive target for RNAi in non-Hodgkin’s lymphoma, Daudi cell line as a model. Leuk Lymphoma 47(9):1941–1948
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2(9):647–656
Davids MS, Pagel JM, Kahl BS, Wierda WG, Miller TP, Gerecitano JF, Rudersdorf NK (2013) Bcl-2 inhibitor ABT-199 (GDC-0199) monotherapy shows anti-tumor activity including complete remissions in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Blood 122(21):872
Dias N, Stein C (2002) Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther 1(5):347–355
Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Little RF (2013) Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med 369(20):1915–1925
Evers MM, Toonen LJ, van Roon-Mom WM (2015) Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev 87:90–103
Farooqi AA, Rehman ZU, Muntane J (2014) Antisense therapeutics in oncology: current status. Onco Targets Ther 7:2035
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Krause K-H (2000) Bcl-2 decreases the free Ca2 + concentration within the endoplasmic reticulum. Proc Natl Acad Sci 97(11):5723–5728
Gallazzi F, Wang Y, Jia F, Shenoy N, Landon LA, Hannink M, Lewis MR (2003) Synthesis of radiometal-labeled and fluorescent cell-permeating peptide-PNA conjugates for targeting the bcl-2 proto-oncogene. Bioconjug Chem 14(6):1083–1095
Galluzzi L, Bravo-San Pedro J, Vitale I, Aaronson S, Abrams J, Adam D, Annicchiarico-Petruzzelli M (2015) Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ 22(1):58–73
Gandhi L, Camidge DR, de Oliveira MR, Bonomi P, Gandara D, Khaira D, Hemken PM (2011) Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 29(7):909–916
Gerard X, Garanto A, Rozet J-M, Collin RW (2016) Antisense oligonucleotide therapy for inherited retinal dystrophies. Retin Degener Dis 854:517–524
Gillies LA, Kuwana T (2014) Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem 115(4):632–640
Goard CA, Schimmer AD (2013) An evidence-based review of obatoclax mesylate in the treatment of hematological malignancies. Core Evid 8:15
Harb W, Lakhani N, Logsdon A, Steigelman M, Smith-Green H, Gaylor S et al (2014) The BCL2 targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 is active in patients with relapsed or refractory non-Hodgkin’s lymphoma. Paper presented at the American Society of hematology annual meeting
Herbst RS, Frankel SR (2004) Oblimersen sodium (Genasense bcl-2 antisense oligonucleotide) a rational therapeutic to enhance apoptosis in therapy of lung cancer. Clin Cancer Res 10(12):4245s–4248s
Izquierdo M (2005) Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Ther 12(3):217–227
Jones LA (1979) Gossypol and some other terpenoids, flavonoids, and phenols that affect quality of cottonseed protein. J Am Oil Chem Soc 56(8):727–730
Kang MH, Reynolds CP (2009) Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15(4):1126–1132
Kelly P, Grabow S, Delbridge A, Adams J, Strasser A (2013) Prophylactic treatment with the BH3 mimetic ABT-737 impedes Myc-driven lymphomagenesis in mice. Cell Death Differ 20(1):57–63
Kirkin V, Joos S, Zörnig M (2004) The role of Bcl-2 family members in tumorigenesis. Biochimica et Biophysica Acta (BBA)-Mol Cell Res 1644(2):229–249
Klasa RJ, Bally MB, Ng R, Goldie JH, Gascoyne RD, Wong FM (2000) Eradication of human non-Hodgkin’s lymphoma in SCID mice by BCL-2 antisense oligonucleotides combined with low-dose cyclophosphamide. Clin Cancer Res 6(6):2492–2500
Large B-Cell Lymphoma. In: ClinicalTrials.gov. National Library of Medicine (US), Bethesda. 2000-(cited 2015 Dec 4). https://clinicaltrials.gov/ct2/show/study/NCT02226965. NLM Identifier:NCT02226965
Liu D, Balkin ER, Jia F, Ruthengael VC, Smith CJ, Lewis MR (2015) Targeted antisense radiotherapy and dose fractionation using a 177 Lu-labeled anti-bcl-2 peptide nucleic acid-peptide conjugate. Nucl Med Biol 42(9):704–710
Maxwell SA, Mousavi-Fard S (2013). Non-Hodgkin’s B-cell lymphoma: advances in molecular strategies targeting drug resistance. Exp Biol Med 238(9):971–990
Mazumder S, Choudhary GS, Al-harbi S, Almasan A (2012) Mcl-1 phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res 72(12):3069–3079
Minn A, Rudin CM, Boise LH, Thompson CB (1995) Expression of bcl-xL can confer a multidrug resistance phenotype. Blood 86(5):1903–1910
Mohammad RM, Wang S, Aboukameel A, Chen B, Wu X, Chen J, Al-Katib A (2005) Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL [(−)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther 4(1):13–21
Mologni L, Nielsen PE, Gambacorti-Passerini C (1999) In vitro transcriptional and translational block of the bcl-2 gene operated by peptide nucleic acid. Biochem Biophys Res Commun 264(2):537–543
Moore VDG, Letai A (2013) BH3 profiling-measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett 332(2):202–205
Nielsen PE (2004) PNA technology. Mol Biotechnol 26(3):233–248
Nielsen PE (2010) Sequence-selective targeting of duplex DNA by peptide nucleic acids. Curr Opin Mol Ther 12(2):184–191
Oki Y, Copeland A, Hagemeister F, Fayad LE, Fanale M, Romaguera J, Younes A (2012) Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood 119(9):2171–2172
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Hajduk PJ (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435(7042):677–681
Packham G, Stevenson FK (2005) Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology 114(4):441–449
Polo JM, Dell’Oso T, Ranuncolo SM, Cerchietti L, Beck D, Da Silva GF, Melnick A (2004) Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med 10(12):1329–1335
Pro B, Leber B, Smith M, Fayad L, Romaguera J, Hagemeister F, Zwiebel J (2008) Phase II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B-cell non-Hodgkin lymphoma. Br J Haematol 143(3):355–360
Profile AR (2002) Augmerosen, Bcl-2 antisense oligonucleotide-genta, GC 3139, Genasense
ProNAi Therapeutics, Inc. A Phase II study of PNT2258 in patients with relapse or refractory diffuse
Reed JC (2008) Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood 111(7):3322–3330
Reed JC, Pellecchia M (2005) Apoptosis-based therapies for hematologic malignancies. Blood 106(2):408–418
Reed JC, Miyashita T, Krajewski S, Takayama S, Aime-Sempe C, Kitada S et al (1996) Bcl-2 family proteins and the regulation of programmed cell death in leukemia and lymphoma. Mol Genet Ther Leuk 84:31–72
Roberts A, Gandhi L, O’Connor O, Rudin C, Khaira D, Xiong H et al (2008) Reduction in platelet counts as a mechanistic biomarker and guide for adaptive dose-escalation in phase I studies of the Bcl-2 family inhibitor ABT-263. Paper presented at the ASCO annual meeting proceedings
Rodrigueza WV, Woolliscroft MJ, Ebrahim A-S, Forgey R, McGovren PJ, Endert G, Gill RD (2014) Development and antitumor activity of a BCL-2 targeted single-stranded DNA oligonucleotide. Cancer Chemother Pharmacol 74(1):151–166
Sagawa Y, Fujitoh A, Nishi H, Ito H, Yudate T, Isaka K (2011) Establishment of three cisplatin-resistant endometrial cancer cell lines using two methods of cisplatin exposure. Tumor Biol 32(2):399–408
Scherr M, Elder A, Battmer K, Barzan D, Bomken S, Ricke-Hoch M, Vormoor J (2014) Differential expression of miR-17 ∼ 92 identifies BCL2 as a therapeutic target in BCR-ABL-positive B-lineage acute lymphoblastic leukemia. Leukemia 28(3):554–565
Sheikhnejad R (2009) MicroDNAs (MIDs) and transcriptional regulation. Nature Precedings http://hdl.handle.net/10101/npre.2009.3931.1
Sioud M (2015) RNA interference: mechanisms, technical challenges, and therapeutic opportunities. Springer, Berlin
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Fairbrother WJ (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208
Tabuchi Y, Matsuoka J, Gunduz M, Imada T, Ono R, Ito M, Takaoka M (2009) Resistance to paclitaxel therapy is related with Bcl-2 expression through an estrogen receptor mediated pathway in breast cancer. Int J Oncol 34(2):313–319
Thomas S, Quinn BA, Das SK, Dash R, Emdad L, Dasgupta S, Pellecchia M (2013) Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets 17(1):61–75
Thomenius MJ, Wang NS, Reineks EZ, Wang Z, Distelhorst CW (2003) Bcl-2 on the endoplasmic reticulum regulates Bax activity by binding to BH3-only proteins. J Biol Chem 278(8):6243–6250
Tolcher AW, Rodrigueza WV, Rasco DW, Patnaik A, Papadopoulos KP, Amaya A, Sooch MP (2014) A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother Pharmacol 73(2):363–371
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Nimmer P (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68(9):3421–3428
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3(11):697–707
Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM (1984) Cloning of the chromosome breakpoint of neoplastic B cells with the t (14; 18) chromosome translocation. Science 226(4678):1097–1099
Tucker CA, Kapanen AI, Chikh G, Hoffman BG, Kyle AH, Wilson IM, Klasa RJ (2008) Silencing Bcl-2 in models of mantle cell lymphoma is associated with decreases in cyclin D1, nuclear factor-κB, p53, bax, and p27 levels. Mol Cancer Ther 7(4):749–758
Vandenberg CJ, Cory S (2013) ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood 121(12):2285–2288
Vickers TA, Crooke ST (2014) Antisense oligonucleotides capable of promoting specific target mRNA reduction via competing RNase H1-dependent and independent mechanisms. PloS one 9(10):e108625
Wang G, Nikolovska-Coleska Z, Yang C-Y, Wang R, Tang G, Guo J, Yang D (2006) Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem 49(21):6139–6142
Watts JK, Corey DR (2012) Silencing disease genes in the laboratory and the clinic. J Pathol 226(2):365–379
Wendt MD (2008) Discovery of ABT-263, a Bcl-family protein inhibitor: observations on targeting a large protein–protein interaction. Expert Opin Drug Discov 3(9):1123–1143
Wu H, Lima WF, Zhang H, Fan A, Sun H, Crooke ST (2004) Determination of the role of the human RNase H1 in the pharmacology of DNA-like antisense drugs. J Biol Chem 279(17):17181–17189
Yang X, Zheng F, Chen J, Gao Q, Lu Y, Wang S, Ma D (2002) Relationship between expression of apoptosis-associated proteins and caspase-3 activity in cisplatin-resistant human ovarian cancer cell line. Ai Zheng 21(12):1288–1291
Zhai D, Jin C, Satterthwait A, Reed J (2006) Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ 13(8):1419–1421
Zhang Y, Lin Y, Min P, Zhang X, Ling X, Guo M, Yang D (2007) A novel pan inhibitor of Bcl-2 and Mcl-1 apogossypolone (ApoG2) with superior stability and improved activity against human leukemia and lymphoma cells. Cancer Res 67(9 Supplement):5182
Acknowledgments
This study was funded by the St. John Hospital and Medical Center Foundation and by Michigan Corporate Relations Network’s (MCRN) Small Company Innovation Program (SCIP). The authors also wish to thank Dr. Mary Walsh for assistance in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ebrahim, A.S., Sabbagh, H., Liddane, A. et al. Hematologic malignancies: newer strategies to counter the BCL-2 protein. J Cancer Res Clin Oncol 142, 2013–2022 (2016). https://doi.org/10.1007/s00432-016-2144-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2144-1