Abstract
Purpose
The present study aims to investigate the role of androgens in controlling the glycolytic metabolism and lactate efflux in prostate cancer (PCa) cells.
Methods
Androgen-responsive LNCaP cells were treated with 5α-dihydrotestosterone (DHT, 10 nM) for 12–48 h, and their glycolytic metabolism, lactate production and viability were analyzed. Intracellular and extracellular levels of glucose and lactate were determined spectrophotometrically, and the expression of glucose transporters (GLUT1/GLUT3), phosphofructokinase 1, lactate dehydrogenase (LDH) and monocarboxylate transporter (MCT4) was analyzed by real-time PCR and Western blot. The enzymatic activity of LDH was determined by means of a colorimetric assay. Experiments were reproduced in androgen-non-responsive DU145 and PC3 cells.
Results
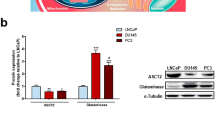
Androgens stimulated glucose consumption in LNCaP cells by increasing the expression of GLUT3, GLUT1 and PFK, which was underpinned by increased cell viability. Accordingly, lactate production by LNCaP cells was enhanced upon DHT stimulation as evidenced by the increased levels of lactate found in cell culture medium. Although LDH enzymatic activity decreased in LNCaP cells treated with DHT, the expression of MCT4 was significantly increased with androgenic treatment, which sustains the increase on lactate export. Glucose consumption and the expression of GLUTs and PFK remained unchanged in DHT-treated DU145 and PC3 cells.
Conclusions
The results obtained establish androgens as modulators of glycolytic metabolism in PCa cells by stimulating glucose consumption, as well as the production and export of lactate, which may represent a crucial issue-driven prostate tumor development. These findings also highlight the importance of PCa therapies targeting AR and metabolism-related proteins.








Similar content being viewed by others
References
Albers MJ et al (2008) Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res 68:8607–8615
Atsumi T et al (2002) High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res 62:5881–5887
Baltazar F, Pinheiro C, Morais-Santos F, Azevedo-Silva J, Queiros O, Preto A, Casal M (2014) Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol Histopathol 29:1511–1524
Brahimi-Horn MC, Bellot G, Pouyssegur J (2011) Hypoxia and energetic tumour metabolism. Curr Opin Genet Dev 21:67–72. doi:10.1016/j.gde.2010.10.006
Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S (2003) Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer 97:2035–2042
Chehtane M, Khaled AR (2010) Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene. Am J Physiol 298:C1560–C1571
Chiche J, Brahimi-Horn MC, Pouyssegur J (2010) Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 14:771–794. doi:10.1111/j.1582-4934.2009.00994.x
Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S (2000) The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J 350(Pt 1):219–227
Effert P, Beniers A, Tamimi Y, Handt S, Jakse G (2004) Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res 24:3057–3064
Emonds KM, Swinnen JV, van Weerden WM, Vanderhoydonc F, Nuyts J, Mortelmans L, Mottaghy FM (2011) Do androgens control the uptake of 18 F-FDG, 11 C-choline and 11 C-acetate in human prostate cancer cell lines? Eur J Nucl Med Mol Imaging 38:1842–1853
Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A (2006) Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. J Physiol 577:433–443. doi:10.1113/jphysiol.2006.115436
Everse J, Kaplan NO (1973) Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol 37:61–133
Feron O (2009) Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 92:329–333. doi:10.1016/j.radonc.2009.06.025
Fischer K et al (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109:3812–3819
Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W (2011) Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol 39:453–463. doi:10.3892/ijo.2011.1055
Gomes IM, Santos CR, Socorro S, Maia CJ (2013) Six transmembrane epithelial antigen of the prostate 1 is down-regulated by sex hormones in prostate cells. Prostate 73:605–613. doi:10.1002/pros.22601
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Hao J et al (2010) Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br J Cancer 103:1008–1018. doi:10.1038/sj.bjc.6605839
Hasawi NA, Alkandari MF, Luqmani YA (2014) Phosphofructokinase: A mediator of glycolytic flux in cancer progression. Crit Rev Oncol Hematol 92:312–321. doi:10.1016/j.critrevonc.2014.05.007
Horii K et al (2007) Androgen-dependent gene expression of prostate-specific antigen is enhanced synergistically by hypoxia in human prostate cancer cells. Mol Cancer Res 5:383–391. doi:10.1158/1541-7786.mcr-06-0226
Jackson DA, Pombo A, Iborra F (2000) The balance sheet for transcription: an analysis of nuclear RNA metabolism in mammalian cells. FASEB J 14:242–254
Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E (2006) Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res 66:632–637
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13:472–482
Larson SM et al (2004) Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med 45:366–373
Li J, Al-Azzawi F (2009) Mechanism of androgen receptor action. Maturitas 63:142–148. doi:10.1016/j.maturitas.2009.03.008
Lin CY, Wu H, Tjeerdema RS, Viant MR (2007) Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 3:55–67
Macheda ML, Rogers S, Best JD (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 202:654–662
Maia C, Santos C, Schmitt F, Socorro S (2009) Regucalcin is under- expressed in human breast and prostate cancers: effect of sex steroid hormones. J Cell Biochem 107:667–676
Martinez-Outschoorn UE et al (2011) Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and Achilles’ heel of tumor cells. Cell Cycle 10:4208–4216. doi:10.4161/cc.10.24.18487
Martins AD et al (2013) Control of Sertoli cell metabolism by sex steroid hormones is mediated through modulation in glycolysis-related transporters and enzymes. Cell Tissue Res 354:861–868. doi:10.1007/s00441-013-1722-7
Massie CE et al (2011) The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 30:2719–2733. doi:10.1038/emboj.2011.158
Mata J, Marguerat S, Bähler J (2005) Post-transcriptional control of gene expression: a genome-wide perspective. Trends Biochem Sci 30:506–514
Moon JS et al (2011) Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. Biochem J 433:225–233. doi:10.1042/BJ20101104
Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274:1393–1418
Nualart F, Garcia MA, Medina RA, Owen GI (2009) Glucose transporters in sex steroid hormone related cancer. Curr Vasc Pharmacol 7:534–548
Oliveira PF et al (2011) Influence of 5alpha-dihydrotestosterone and 17beta-estradiol on human Sertoli cells metabolism. Int J Androl 34:e612–e620. doi:10.1111/j.1365-2605.2011.01205.x
Oyama N et al (2001) FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun 22:963–969
Pertega-Gomes N et al (2011) Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer 11:312
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 29:e45
Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F (2012) Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr 44:127–139
Sato K, Iemitsu M, Aizawa K, Ajisaka R (2008) Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab 294:E961–E968
Schweizer L et al (2008) The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC Cell Biol 9:4. doi:10.1186/1471-2121-9-4
Sharova LV, Sharov AA, Nedorezov T, Piao Y, Shaik N, Ko MS (2009) Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res 16:45–58. doi:10.1093/dnares/dsn030
Swanson MG et al (2006) Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn Reson Med 55:1257–1264
Swietach P, Vaughan-Jones RD, Harris AL (2007) Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 26:299–310
Tessem MB et al (2008) Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn Reson Med 60:510–516
Vaz CV, Alves MG, Marques R, Moreira PI, Oliveira PF, Maia CJ, Socorro S (2012) Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int J Biochem Cell Biol 44:2077–2084. doi:10.1016/j.biocel.2012.08.013
Wang D, Tindall DJ (2011) Androgen action during prostate carcinogenesis. Methods Mol Biol 776:25–44. doi:10.1007/978-1-61779-243-4_2
Warburg O, Wind F, Negelein E (1926) Ueber den Stoffwechsel von Tumoren im korper. J Mol Med 5:829–832
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530
Acknowledgments
This work was partially supported by the Pluriannual Programme of Portuguese Foundation for Science and Technology (FCT) under Program COMPETE (PEst-OE/SAU/UI0709/2014). Pedro F. Oliveira was financed by FCT under the program Ciência 2008. Cátia Vaz, Ricardo Marques and Marco Alves were funded by FCT fellowships (SFRH/BD/70316/2010, SFRH/BD/66875/2009 and SRFH/BPD/80451/2011, respectively).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaz, C.V., Marques, R., Alves, M.G. et al. Androgens enhance the glycolytic metabolism and lactate export in prostate cancer cells by modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes. J Cancer Res Clin Oncol 142, 5–16 (2016). https://doi.org/10.1007/s00432-015-1992-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1992-4