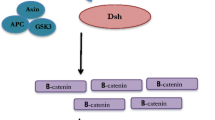
Abstract
Purpose
This review aims to summarize the evidence that Notch signaling is associated with prostate development, tumorigenesis and prostate tumor progression.
Methods
Studies in PubMed database were searched using the keywords of Notch signaling, prostate development and prostate cancer. Relevant literatures were identified and summarized.
Results
The Notch pathway plays an important role in determining cell fate, proliferation, differentiation and apoptosis. Recent findings have highlighted the involvement of Notch signaling in prostate development and in the maintenance of adult prostate homeostasis. Aberrant Notch expression in tissues leads to dysregulation of Notch functions and promotes various neoplasms, including prostate cancer. High expression of Notch has been implicated in prostate cancer, and its expression increases with higher cancer grade. However, the precise role of Notch in prostate cancer has yet to be clearly defined. The roles of Notch either as an oncogene or tumor suppressor in prostate cancer hallmarks such as cell proliferation, apoptosis and anoikis, hypoxia, migration and invasion, angiogenesis as well as the correlation with metastasis are therefore discussed.
Conclusions
Notch signaling is a complicated signaling pathway in modulating prostate development and prostate cancer. Understanding and manipulating Notch signaling could therefore be of potential therapeutic value in combating prostate cancer.



Similar content being viewed by others
References
Abate-Shen C, Shen MM (2000) Molecular genetics of prostate cancer. Genes Dev 14:2410–2434
Ables JL, Breunig JJ, Eisch AJ, Rakic P (2011) Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 12:269–283. doi:10.1038/nrn3024
Alana L et al (2014) Prostate tumor OVerexpressed-1 (PTOV1) down-regulates HES1 and HEY1 Notch targets genes and promotes prostate cancer progression. Mol Cancer 13:74. doi:10.1186/1476-4598-13-74
Allenspach EJ, Maillard I, Aster JC, Pear WS (2002) Notch signaling in cancer. Cancer Biol Ther 1:466–476
Andersen P, Uosaki H, Shenje LT, Kwon C (2012) Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol 22:257–265. doi:10.1016/j.tcb.2012.02.003
Andersson ER, Lendahl U (2014) Therapeutic modulation of Notch signalling—Are we there yet? Nat Rev Drug Discov 13:357–378. doi:10.1038/nrd4252
Aoyagi K, Shima I, Wang M, Hu Y, Garcia FU, Stearns ME (1998) Specific transcription factors prognostic for prostate cancer progression. Clin Cancer Res 4:2153–2160
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Baladron V et al (2005) dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res 303:343–359. doi:10.1016/j.yexcr.2004.10.001
Belandia B, Powell SM, Garcia-Pedrero JM, Walker MM, Bevan CL, Parker MG (2005) Hey1, a mediator of Notch signaling, is an androgen receptor corepressor. Mol Cell Biol 25:1425–1436. doi:10.1128/MCB.25.4.1425-1436.2005
Benedit P et al (2001) PTOV1, a novel protein overexpressed in prostate cancer containing a new class of protein homology blocks. Oncogene 20:1455–1464. doi:10.1038/sj.onc.1204233
Berman DM et al (2004) Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol 267:387–398. doi:10.1016/j.ydbio.2003.11.018
Bin Hafeez B et al (2009) Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin Cancer Res 15:452–459. doi:10.1158/1078-0432.CCR-08-1631
Blackwood JK et al (2011) In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J Pathol 225:181–188. doi:10.1002/path.2965
Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S (1997) Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90:281–291
Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689. doi:10.1038/nrm2009
Bray SJ, Takada S, Harrison E, Shen SC, Ferguson-Smith AC (2008) The atypical mammalian ligand delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev Biol 8:11. doi:10.1186/1471-213X-8-11
Brou C et al (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease. TACE Mol Cell 5:207–216
Bush G, diSibio G, Miyamoto A, Denault JB, Leduc R, Weinmaster G (2001) Ligand-induced signaling in the absence of furin processing of Notch1. Dev Biol 229:494–502. doi:10.1006/dbio.2000.9992
Carvalho FL, Simons BW, Eberhart CG, Berman DM (2014) Notch signaling in prostate cancer: a moving target. Prostate 74:933–945. doi:10.1002/pros.22811
Ceder JA, Jansson L, Helczynski L, Abrahamsson PA (2008) Delta-like 1 (Dlk-1), a novel marker of prostate basal and candidate epithelial stem cells, is downregulated by Notch signalling in intermediate/transit amplifying cells of the human prostate. Eur Urol 54:1344–1353. doi:10.1016/j.eururo.2008.03.006
Chandran UR et al (2007) Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 7:64. doi:10.1186/1471-2407-7-64
Chang C, Lee SO, Yeh S, Chang TM (2014) Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 33:3225–3234. doi:10.1038/onc.2013.274
Choi JH, Park JT, Davidson B, Morin PJ, Shih Ie M, Wang TL (2008) Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res 68:5716–5723. doi:10.1158/0008-5472.CAN-08-0001
Choi N, Zhang B, Zhang L, Ittmann M, Xin L (2012) Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21:253–265. doi:10.1016/j.ccr.2012.01.005
Cooper CR et al (2003) Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 97:739–747. doi:10.1002/cncr.11181
Cunha GR, Hayward SW, Wang YZ (2002) Role of stroma in carcinogenesis of the prostate. Differentiation 70:473–485. doi:10.1046/j.1432-0436.2002.700902.x
Danza G et al (2012) Notch signaling modulates hypoxia-induced neuroendocrine differentiation of human prostate cancer cells. Mol Cancer Res 10:230–238. doi:10.1158/1541-7786.MCR-11-0296
Danza G et al (2013) Notch3 is activated by chronic hypoxia and contributes to the progression of human prostate cancer. Int J Cancer 133:2577–2586. doi:10.1002/ijc.28293
Domingo-Domenech J et al (2012) Suppression of acquired docetaxel resistance in prostate cancer through depletion of Notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 22:373–388. doi:10.1016/j.ccr.2012.07.016
Donjacour AA, Thomson AA, Cunha GR (2003) FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol 261:39–54
D’Souza B, Meloty-Kapella L, Weinmaster G (2010) Canonical and non-canonical Notch ligands. Curr Top Dev Biol 92:73–129. doi:10.1016/S0070-2153(10)92003-6
Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL (2010) Genomic profiling of tumor initiating prostatospheres. BMC Genom 11:324. doi:10.1186/1471-2164-11-324
Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J (1991) TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66:649–661
Fischer A, Gessler M (2007) Delta-Notch–and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res 35:4583–4596. doi:10.1093/nar/gkm477
Fleming RJ (1998) Structural conservation of Notch receptors and ligands. Semin Cell Dev Biol 9:599–607. doi:10.1006/scdb.1998.0260
Flores AN, McDermott N, Meunier A, Marignol L (2014) NUMB inhibition of NOTCH signalling as a therapeutic target in prostate cancer. Nat Rev Urol 11:499–507. doi:10.1038/nrurol.2014.195
Fraering PC et al (2004) Purification and characterization of the human gamma-secretase complex. Biochemistry 43:9774–9789. doi:10.1021/bi0494976
Frank SB, Miranti CK (2013) Disruption of prostate epithelial differentiation pathways and prostate cancer development. Front Oncol 3:273. doi:10.3389/fonc.2013.00273
Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA (2003) Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol 264:352–362
Gaiano N, Fishell G (2002) The role of Notch in promoting glial and neural stem cell fates. Annu Rev Neurosci 25:471–490. doi:10.1146/annurev.neuro.25.030702.130823
Gaisa NT et al (2011) Clonal architecture of human prostatic epithelium in benign and malignant conditions. J Pathol 225:172–180. doi:10.1002/path.2959
Gao D et al (2014) Organoid cultures derived from patients with advanced prostate cancer. Cell 159:176–187. doi:10.1016/j.cell.2014.08.016
Gelmann EP (2002) Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015
Grego-Bessa J et al (2007) Notch signaling is essential for ventricular chamber development. Dev Cell 12:415–429. doi:10.1016/j.devcel.2006.12.011
Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD (2005) BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol 288:334–347. doi:10.1016/j.ydbio.2005.08.018
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, Martinez Arias A (2005) Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development 132:1819–1830. doi:10.1242/dev.01724
Heer R, Douglas D, Mathers ME, Robson CN, Leung HY (2004) Fibroblast growth factor 17 is over-expressed in human prostate cancer. J Pathol 204:578–586. doi:10.1002/path.1668
Hu YY, Zheng MH, Zhang R, Liang YM, Han H (2012) Notch signaling pathway and cancer metastasis. Adv Exp Med Biol 727:186–198. doi:10.1007/978-1-4614-0899-4_14
Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R (2010) Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci 30:3489–3498. doi:10.1523/JNEUROSCI.4987-09.2010
Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255. doi:10.1002/jcp.10208
Joutel A et al (1996) Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383:707–710. doi:10.1038/383707a0
Kalin TV et al (2006) Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res 66:1712–1720. doi:10.1158/0008-5472.CAN-05-3138
Kannan S et al (2013) Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med 210:321–337. doi:10.1084/jem.20121527
Kao HY et al (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev 12:2269–2277
Karantanos T, Corn PG, Thompson TC (2013) Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32:5501–5511. doi:10.1038/onc.2013.206
Kidd S, Lieber T (2002) Furin cleavage is not a requirement for Drosophila Notch function. Mech Dev 115:41–51
Kidd S, Kelley MR, Young MW (1986) Sequence of the Notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol 6:3094–3108
Kwon OJ et al (2014) Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat Commun 5:4416. doi:10.1038/ncomms5416
Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W (2001) Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol 232:301–314. doi:10.1006/dbio.2001.0187
Lavery DN, Villaronga MA, Walker MM, Patel A, Belandia B, Bevan CL (2011) Repression of androgen receptor activity by HEYL, a third member of the Hairy/Enhancer-of-split-related family of Notch effectors. J Biol Chem 286:17796–17808. doi:10.1074/jbc.M110.198655
Lawson DA, Witte ON (2007) Stem cells in prostate cancer initiation and progression. J Clin Invest 117:2044–2050. doi:10.1172/JCI32810
Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A (2007) Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through slug-induced repression of E-cadherin. J Exp Med 204:2935–2948. doi:10.1084/jem.20071082
Li L et al (1997) Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16:243–251. doi:10.1038/ng0797-243
Li JL et al (2007) Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 67:11244–11253. doi:10.1158/0008-5472.CAN-07-0969
Liotta LA, Kohn E (2004) Anoikis: cancer and the homeless cell. Nature 430:973–974. doi:10.1038/430973a
Lobry C et al (2013) Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J Exp Med 210:301–319. doi:10.1084/jem.20121484
Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A (1998) The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA 95:8108–8112
Long RM, Morrissey C, Fitzpatrick JM, Watson RW (2005) Prostate epithelial cell differentiation and its relevance to the understanding of prostate cancer therapies. Clin Sci (Lond) 108:1–11. doi:10.1042/CS20040241
Marker PC, Donjacour AA, Dahiya R, Cunha GR (2003) Hormonal, cellular, and molecular control of prostatic development. Dev Biol 253:165–174
Masuda S, Izpisua Belmonte JC (2014) A recipe for targeted therapy in prostate cancer. Nat Rev Urol 11:419. doi:10.1038/nrurol.2013.110-c1
McDonnell TJ et al (1992) Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 52:6940–6944
Miele L (2006) Notch signaling. Clin Cancer Res 12:1074–1079. doi:10.1158/1078-0432.CCR-05-2570
Miki J, Rhim JS (2008) Prostate cell cultures as in vitro models for the study of normal stem cells and cancer stem cells. Prostate Cancer Prostatic Dis 11:32–39. doi:10.1038/sj.pcan.4501018
Morgan TH (1917) The theory of the gene. Am Nat 51:513–544
Mulligan P et al (2011) A SIRT1-LSD1 corepressor complex regulates Notch target gene expression and development. Mol Cell 42:689–699. doi:10.1016/j.molcel.2011.04.020
Mumm JS, Kopan R (2000) Notch signaling: from the outside in. Dev Biol 228:151–165. doi:10.1006/dbio.2000.9960
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 100:14920–14925. doi:10.1073/pnas.2436557100
Nagel AC, Krejci A, Tenin G, Bravo-Patino A, Bray S, Maier D, Preiss A (2005) Hairless-mediated repression of Notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol Cell Biol 25:10433–10441. doi:10.1128/MCB.25.23.10433-10441.2005
Nantermet PV et al (2004) Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J Biol Chem 279:1310–1322. doi:10.1074/jbc.M310206200
Nicolas M et al (2003) Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 33:416–421. doi:10.1038/ng1099
Oda T et al (1997) Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 16:235–242. doi:10.1038/ng0797-235
Oktem G et al (2014) Expression profiling of stem cell signaling alters with spheroid formation in CD133/CD44 prostate cancer stem cells. Oncol Lett 7:2103–2109. doi:10.3892/ol.2014.1992
Orr B, Grace OC, Vanpoucke G, Ashley GR, Thomson AA (2009) A role for Notch signaling in stromal survival and differentiation during prostate development. Endocrinology 150:463–472. doi:10.1210/en.2008-0383
Orr B et al (2013) Reduction of pro-tumorigenic activity of human prostate cancer-associated fibroblasts using Dlk1 or SCUBE1. Dis Model Mech 6:530–536. doi:10.1242/dmm.010355
Ortega M, Bhatnagar H, Lin AP, Wang L, Aster JC, Sill H, Aguiar RC (2014) A microRNA-mediated regulatory loop modulates NOTCH and MYC oncogenic signals in B- and T-cell malignancies. Leukemia. doi:10.1038/leu.2014.302
Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C (2012) Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol 14:1131–1138. doi:10.1038/ncb2600
Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D (2013) Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med 19:1054–1060. doi:10.1038/nm.3259
Park JT et al (2006) Notch3 gene amplification in ovarian cancer. Cancer Res 66:6312–6318. doi:10.1158/0008-5472.CAN-05-3610
Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W (1999) Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol 209:28–39. doi:10.1006/dbio.1999.9229
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Rangarajan A et al (2001) Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20:3427–3436. doi:10.1093/emboj/20.13.3427
Raouf A et al (2008) Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3:109–118. doi:10.1016/j.stem.2008.05.018
Reiter RE et al (1998) Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA 95:1735–1740
Ridgway J et al (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444:1083–1087. doi:10.1038/nature05313
Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L (2008) Rational targeting of Notch signaling in cancer. Oncogene 27:5124–5131. doi:10.1038/onc.2008.226
Robbins J, Blondel BJ, Gallahan D, Callahan R (1992) Mouse mammary tumor gene int-3: a member of the Notch gene family transforms mammary epithelial cells. J Virol 66:2594–2599
Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 105:6392–6397. doi:10.1073/pnas.0802047105
Sainson RC, Harris AL (2007) Anti-Dll4 therapy: Can we block tumour growth by increasing angiogenesis? Trends Mol Med 13:389–395. doi:10.1016/j.molmed.2007.07.002
Salta E, Lau P, Sala Frigerio C, Coolen M, Bally-Cuif L, De Strooper B (2014) A self-organizing miR-132/Ctbp2 circuit regulates bimodal Notch signals and glial progenitor fate choice during spinal cord maturation. Dev Cell 30:423–436. doi:10.1016/j.devcel.2014.07.006
Salvati M et al (2005) Brain metastasis from prostate cancer. Report of 13 cases and critical analysis of the literature. J Exp Clin Cancer Res 24:203–207
Santagata S et al (2004) JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res 64:6854–6857. doi:10.1158/0008-5472.CAN-04-2500
Santamaria A et al (2003) PTOV-1, a novel protein overexpressed in prostate cancer, shuttles between the cytoplasm and the nucleus and promotes entry into the S phase of the cell division cycle. Am J Pathol 162:897–905. doi:10.1016/S0002-9440(10)63885-0
Scorey N, Fraser SP, Patel P, Pridgeon C, Dallman MJ, Djamgoz MB (2006) Notch signalling and voltage-gated Na + channel activity in human prostate cancer cells: independent modulation of in vitro motility. Prostate Cancer Prostatic Dis 9:399–406. doi:10.1038/sj.pcan.4500894
Sethi S, Macoska J, Chen W, Sarkar FH (2010) Molecular signature of epithelial–mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res 3:90–99
Shen MM, Abate-Shen C (2010) Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 24:1967–2000. doi:10.1101/gad.1965810
Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ (2001) Dynamics of Notch expression during murine prostate development and tumorigenesis. Cancer Res 61:7291–7297
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29. doi:10.3322/caac.20138
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30. doi:10.3322/caac.21166
Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, Ball DW (2001) Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 61:3200–3205
Swanson GP, Thompson IM, Basler J (2006) Current status of lymph node-positive prostate cancer: incidence and predictors of outcome. Cancer 107:439–450. doi:10.1002/cncr.22034
Thurston G, Noguera-Troise I, Yancopoulos GD (2007) The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer 7:327–331. doi:10.1038/nrc2130
Valdez JM et al (2012) Notch and TGFbeta form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity. Cell Stem Cell 11:676–688. doi:10.1016/j.stem.2012.07.003
Valve EM, Nevalainen MT, Nurmi MJ, Laato MK, Martikainen PM, Harkonen PL (2001) Increased expression of FGF-8 isoforms and FGF receptors in human premalignant prostatic intraepithelial neoplasia lesions and prostate cancer. Lab Invest 81:815–826
van Es JH et al (2005) Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435:959–963. doi:10.1038/nature03659
Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA (1992) Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 52:6182–6187
Wang Y, Hayward S, Cao M, Thayer K, Cunha G (2001) Cell differentiation lineage in the prostate. Differentiation 68:270–279
Wang XD, Shou J, Wong P, French DM, Gao WQ (2004) Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem 279:24733–24744. doi:10.1074/jbc.M401602200
Wang XD et al (2006) Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol 290:66–80. doi:10.1016/j.ydbio.2005.11.009
Wang Z, Li Y, Banerjee S, Sarkar FH (2008) Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res 28:3621–3630
Wang X et al (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461:495–500. doi:10.1038/nature08361
Wang Z et al (2010) Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem 109:726–736. doi:10.1002/jcb.22451
Wang Z et al (2011) Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J Cell Biochem 112:78–88. doi:10.1002/jcb.22770
Webber J et al (2014) Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Mol Cell Proteomics 13:1050–1064. doi:10.1074/mcp.M113.032136
Weinmaster G, Roberts VJ, Lemke G (1991) A homolog of Drosophila Notch expressed during mammalian development. Development 113:199–205
Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S (1985) Nucleotide sequence from the neurogenic locus Notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43:567–581
Whelan JT, Kellogg A, Shewchuk BM, Hewan-Lowe K, Bertrand FE (2009) Notch-1 signaling is lost in prostate adenocarcinoma and promotes PTEN gene expression. J Cell Biochem 107:992–1001. doi:10.1002/jcb.22199
Wu CT et al (2007) Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA 104:12679–12684. doi:10.1073/pnas.0704940104
Wu X et al (2011) Differentiation of the ductal epithelium and smooth muscle in the prostate gland are regulated by the Notch/PTEN-dependent mechanism. Dev Biol 356:337–349. doi:10.1016/j.ydbio.2011.05.659
Yatim A et al (2012) NOTCH1 nuclear interactome reveals key regulators of its transcriptional activity and oncogenic function. Mol Cell 48:445–458. doi:10.1016/j.molcel.2012.08.022
Yong T, Sun A, Henry MD, Meyers S, Davis JN (2011) Down regulation of CSL activity inhibits cell proliferation in prostate and breast cancer cells. J Cell Biochem 112:2340–2351. doi:10.1002/jcb.23157
Yu Y, Zhang Y, Guan W, Huang T, Kang J, Sheng X, Qi J (2014) Androgen receptor promotes the oncogenic function of overexpressed Jagged1 in prostate cancer by enhancing cyclin B1 expression via Akt phosphorylation. Mol Cancer Res 12:830–842. doi:10.1158/1541-7786.MCR-13-0545
Zayzafoon M, Abdulkadir SA, McDonald JM (2004) Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem 279:3662–3670. doi:10.1074/jbc.M308158200
Zhang Y, Wang Z, Ahmed F, Banerjee S, Li Y, Sarkar FH (2006) Down-regulation of Jagged-1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int J Cancer 119:2071–2077. doi:10.1002/ijc.22077
Zhu H, Zhou X, Redfield S, Lewin J, Miele L (2013) Elevated Jagged-1 and Notch-1 expression in high grade and metastatic prostate cancers. Am J Transl Res 5:368–378
Acknowledgments
This work was supported by grants from the Zhejiang Provincial Natural Science Foundation (Y2111329), Zhejiang Provincial Medicines Health Technology Project (2011KYB066), Zhejiang Provincial Traditional Medicine Technology Project (2011ZB099), Hangzhou Health Technology Project (20110833B05) and the Zhejiang Provincial Medical Association Clinical Research Funding Project (2012ZYC-A35).
Conflict of interest
The authors declare that they have no known conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, G., Ma, L., Meng, Q. et al. Notch signaling in the prostate: critical roles during development and in the hallmarks of prostate cancer biology. J Cancer Res Clin Oncol 142, 531–547 (2016). https://doi.org/10.1007/s00432-015-1946-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1946-x