Abstract
Purpose
Patients with advanced hepatocellular carcinoma (HCC) have shown to benefit from tamoxifen treatment. The mechanisms of tamoxifen action in HCC, however, are not yet clearly understood. Results from studies on the human hepatoblastoma cell line HepG2 provide evidence that estrogen-receptor-α-independent antiproliferative actions of tamoxifen in HCC are mediated by the suppression of telomerase activity [5].
Materials and methods
We investigate the pathway of the tamoxifen-induced down-regulation of telomerase activity, using HepG2 cells incubated over 24 h or 48 h in the presence of 20 μM tamoxifen.
Results
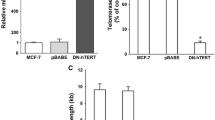
The transcriptional levels of the three telomerase core components—human telomerase RNA (hTR), human telomerase reverse transcriptase (hTERT) (all variants), and telomerase-associated protein (TP1)—did not change during tamoxifen treatment, as revealed by RT-PCR analysis. Furthermore, the hTERT splice pattern was not shifted from the active full-length variant (+α/+β) to the inactive deletion variants (−α; −β; −α/−β) and the level of the 120 kDa hTERT full-length protein remained constant, as shown by Western blot analysis. Protein kinase C (PKC) activity has been suggested to be crucial for post-translational up-regulation of telomerase activity. In HepG2 cells, we observed a tamoxifen-induced suppression of the total protein kinase C (PKC) activity (cytosolic and membrane-bound). Inhibition of PKC with bisindolylmaleimide I resulted in a reduction of telomerase activity, as revealed by TRAP-assay. α-tocopherol (vitamin E) diminished the effects of tamoxifen on PKC-acivity as well as on telomerase activity.
Conclusions
We conclude that the tamoxifen-induced decrease of telomerase activity in HepG2 cells is mediated post-translationally via suppression of PKC-activity.






Similar content being viewed by others
References
Aisner DL, Wright WE, Shay JW (2002) Telomerase regulation: not just flipping the switch. Curr Opin Genet Dev 12:80–85
Aldous WK, Marean AJ, DeHart MJ, Matej LA, Moore KH (1999) Effects of tamoxifen on telomerase activity in breast carcinoma cell lines. Cancer 85:1523–1529
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–335
Brandt S, Heller H, Schuster K-D, Grote J (2004) Tamoxifen induces suppression of cell viability and apoptosis in the human hepatoblastoma cell line HepG2 via down-regulation of telomerase activity. Liver 24:46–54
Cao Y, Li H, Deb S, Liu JP (2002) TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene 21:3130–3138
Cheng AL, Chuang SE, Fine RL, Yeh KH, Liao CM, Lay JD, Chen DS (1998) Inhibition of the membrane translocation and activation of protein kinase C, and potentiation of doxorubicin-induced apoptosis of hepatocellular carcinoma cells by tamoxifen. Biochem Pharmacol 55:523–531
Colgin LM, Wilkinson C, Englezou A, Kilian A, Robinson MO, Reddel RR (2000) The hTERTα slice variant is a dominant negative inhibitor of telomerase activity. Neoplasia 2:433–440
Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S (1992) Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 11:1921–1999
Deacon EM, Pongracz J, Griffiths G, Lord JM (1997) Isoenzyms of protein kinase C differential involvement in apoptosis and pathogenesis. Mol Pathol 50:124–131
Feng J, Funk WD, Wang SS (1995) The RNA component of human telomerase. Science 269:1236–1241
Gelmann E (1997) Tamoxifen for the treatment of malignancies other than breast and endomerial carcinoma. Semin Oncol 24:165–170
Goldstein S (1990) Replicative senescence: the human fibroblast comes of age. Science 249:1129–1133
Gundimeda U, Chen ZH, Gopalakrishna R (1996) Tamoxifen modulates protein kinase C via oxidative stress in estrogen receptor-negative breast cancer cells. J Biol Chem 271:13504–13514
Harley CB (1997) Aging of cultured human skin fibroblasts. In: Pollard JW, Walker JM (eds) Methods in molecular biology, vol. 5. Humana, Clifton, NJ, pp 25–32
Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246:629–634
Helder MN, Wisman GB, Van der Zee GJ (2002) Telomerase and telomers: from basic biology to cancer treatment. Cancer Invest 20:82–101
Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR (1999) Inhibition of human telomerase in immortal cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA 96:14276–14281
Hoekstra R, Chamuleau RAFM (2002) Recent developments on human cell lines for the bioartificial liver. Int J Artif Org 25:182–191
Jiang SY, Shyu RY, Yeh MY, Jordan VC (1995) Tamoxifen inhibits hepatoma cell growth through an estrogen receptor independent mechanism. J Hepatol 23:712–719
Jun-Ping L (1999) Studies of the molecular mechanisms in regulation of telomerase activity. FASEB J 13:2091–104
Karlseder J, Broccoli D, Daui Y, Hardy S, Lange T (1999) p53-and ATM-dependent apoptosis induced by telomerses lacking TRF2. Science 283:1321–1325
Kilian A, Bowtell DD, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA (1997) Isolation of a canditate human telomerase catalytic subunit gene, which reveales complex slicing patterns in different cell types. Hum Mol Genet 6:2011–2019
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GMN, Wright WE, Weinrich SL Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015
Kim YW, Hur SY, Kim TE, Lee JM, Namkoong SE, Ki IK, Kim JW (2001) Protein kinase C modulates telomerase activity in human cervical cancer cells. Exp Mol Med 33:156–163
Kim JA, Young SK, Jung MW, Lee SH, Yong SL (1999) Involvement of Ca2+ influx in the mechanism of tamoxifen induced apoptosis in HepG2 human hepatoblastoma cells. Cancer Lett 147:115–123
Lee YS, Kang YS, Lee SH (2000) Role of NADPH oxidase in tamoxifen-induced generation of reactive oxygen species and apoptosis in HepG2 human hepatoblastoma cells. Cell Death Differ 7:925–932
Linger J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR (1997) Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276:561–567
Liu JP (2001) Telomerase, agening and disease. In: Mattson MP, Pandita T (eds) Elsevier Science, Netherlands, pp 33–59
Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Linger J, Harley CB, Chech TR (1997) Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955–959
Mario M, Distefano E, Trantalance A, Smith CL (2001) Estradiol-induced IP(3) mediates the estrogen receptor activity expressed in human cells. Mol Cell Endocrinol 182:19–26
Olovnikov AM (1973) A thery of marginotomy: the incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomen. J Thero Biol 41:181–190
Smith LL, Coller HA, Roberts JM (2003) Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 5:474–479
Stewart N, Bacchetti S (1991) Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology 180:49–57
Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR (1998) Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate slicing of hTERT transcripts. Cancer Res 58:4168–4172
Ulaner GA, Hu JF, Vu TH, Oruganti H, Giudice LC, Hoffman AR (2000) Regulation of telomerase by alternate splicing of human telomerase reverse transcriptase (hTERT) in normal and neoplastic ovary, endometrium and myometrium. Int J Cancer 85:330–335
Vaziri H, Benchimol S (1998) Reconstitution of telomerase activity in normal human cells leads to elongation of telomers and extended replicative life span. Curr Biol 8:279–282
Von Zglinicki T, Saretzki G, Docke W, Lotze C (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 220:186–193
Wang Z, Kyo S, Maida Y, Takakura M, Tanaka M, Yatabe N (2002) Tamoxifen regulates human telomerase reverse transcriptase (hTERT) gene expression differently in breast and endometrial cancer cells. Oncogene 21:3517–3524
Wick M, Zubov D, Hagen G (1999) Genomic organisation and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene 232:97–106
Wright WE, Pereira-Smith OM, Shay JW (1989) Reversible cellulare senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol 9:3088–3092
Wyllie FS, Jones CJ, Skinner JW, Haughton MF, Wallis C, Waynford-Thomas D, et al (2000) Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nature Genet 24:16–17
Xiang H, Wang J, Mao YW, Li DW (2000) hTERT can function with rabbit telomerase RNA: regulation of gene expression and attenuation of apoptosis. Biochem Biophys Res Commun 278:503–510
Yajima T, Yagihashi A, Kameshima H, Furuya D, Kobayashi D, Hirata K, Watanabe N (2000) Establishment of quantitative reverse transcription-polymerase chain reaction assays for human telomerase-associated genes. Clin Chim Acta 290:117–127
Yi X, Shay JW, Woodring EW (2001) Quantification of telomerase components and hTERT mRNA splicing patterns in immortal human cells. Nucleic Acids Res 29:4818–4825
Yi X, Tesmer VM, Savre-Train I, Shay JW, Wright WE (1999) Both transcriptional and postranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol 19:3989–3997
Zhu H, Fu W, Mattson MP (2000) The catalytic subunit of telomerase protects neurons against amyloid β-peptide-induced apoptosis. J Neurochem. 75:117–127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandt, S., Heller, H., Schuster, KD. et al. The tamoxifen-induced suppression of telomerase activity in the human hepatoblastoma cell line HepG2: a result of post-translational regulation. J Cancer Res Clin Oncol 131, 120–128 (2005). https://doi.org/10.1007/s00432-004-0589-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-004-0589-0