Abstract
A growing body of research provides compelling evidence that in rats 50-kHz USVs are a form of expression of positive emotions. Context-induced 50-kHz USVs emission is variable among rats, indicating individual differences in contextual response bound up with pharmacological reward. The aims of this study were to: extract the most important neurotransmitters related to context-induced conditioned 50-kHz USVs response; find biological basis of existing inter-individual differences in context-induced conditioned 50-kHz USVs response; create a model of all-to-all neurotransmitters correlations. The data collected here confirms that re-exposure to the context of morphine administration after the withdrawal period increases the level of 50-kHz USVs and this contextual response is associated with elevated serotonin concentrations in amygdala, hippocampus and mPFC and with increased Glu/Gln ratio in nucleus accumbens. The concentration of serotonin increases simultaneously in amygdala, nucleus accumbens and hippocampus. Moreover, 5-HT concentration in amygdala is bound up with glutamate level in this structure as well as in hippocampus. Furthermore, Glu/Gln ratio in nucleus accumbens has strong associations with Glu/Gln ratio simultaneously in VTA, amygdala, striatum and hippocampus. All-to-all-analysis indicate that concentration of glutamate in hippocampus is proportional to glutamate in VTA and GABA concentration in the hippocampus. We have also demonstrated that Glu/GABA ratio in VTA and amygdala was elevated after post withdrawal re-exposure to the pharmacological reward paired context. Presented analysis indicates a strong correlation between serotonergic and glutamatergic systems in context-induced conditioned response. The strength of this co-transmission correlates with the number of 50-kHz USVs emitted in response to morphine-paired context.
Similar content being viewed by others
Introduction
A modern behavioral tool that most effectively determines the emotional states of rats is the registration and analysis of ultrasonic vocalizations (USVs). It allows both identifying individual differences in processing information about the reward as well as reflecting, to a large extent, the level of individual motivation. It is well established that rats emit two district ultrasonic vocalizations patterns (USVs) related to separate emotional tinge: long 22-kHz alarm calls when processing negative emotions and 50-kHz when processing positive emotions (Panksepp and Burgdorf 2003; Brudzynski 2013a, b; Burgdorf et al. 2017). Representations of these sounds are also present in rats’ social interactions (Knutson et al. 1998; Brudzynski and Pniak 2002; Hamed et al. 2009, 2015). The mesolimbic reward system is highly involved in the production of 50-kHz ultrasonic vocalizations (USVs) in rats (Knutson et al. 1999; Burgdorf et al. 2001, 2007). Large number of studies indicate that the most potent pharmacological agent that induces 50-kHz USVs in rats is amphetamine (Burgdorf et al. 2001; Thompson et al. 2006; Wang et al. 2008; Ahrens et al. 2009; Wright et al. 2010; Brudzynski et al. 2011; Simola et al. 2012; Taracha et al. 2012). In a recent study we conducted non-parametric analysis of neurochemical effects showing that re-exposure to amphetamine induces neurochemical changes in several brain areas (Hamed et al. 2016). We demonstrated that increased concentration of noradrenaline in the nucleus accumbens strongly correlated with the number of 50-kHz USVs (Hamed et al. 2016). Another set of pharmacological experiments demonstrated that noradrenaline related mechanisms are highly involved in 50-kHz USVs emission (Wright et al. 2012a). Previous studies have established that intracerebral injection of glutamate may induce 50-kHz USVs, suggesting that glutamatergic system is also involved in production of these sounds (Fu and Brudzynski 1994; Wintink and Brudzynski 2001). Additionally, administration of the amphetamine with MK-801 (NMDA antagonist) suppressed emission of the 50-kHz USVs compared to amphetamine treated animals, suggesting that glutamatergic system may be highly engaged in 50-kHz USVs emission (Costa et al. 2015). Co-administration of those two substances suppressed likewise context-induced conditioned 50-kHz USVs when animals were re-exposed to the drug-paired chamber (Costa et al. 2015). Moreover, there are evidence showing that antagonism of 5-HT 2C or the κ-opioid receptors increases emission of 50-kHz USVs (Wöhr et al. 2015; Hamed et al. 2015). Furthermore, agonist of 5-HT2C receptor suppressed amphetamine induced sounds in this frequency band (Wöhr et al. 2015).
A growing body of research provides compelling evidence that 50-kHz USVs (Fig. 1) are a form of expression of positive emotions. However, pharmacological studies indicate that processing of positive emotions is not always accompanied by the 50-kHz USVs in rats. In case of morphine treatment, higher doses of this addictive drug (10 mg/kg) decreased the sounds triggered by social interaction (Hamed et al. 2015). It has been also demonstrated that MDMA, which induces huge arousal (excitation) in humans, did not evoke an increase in total number of 50-kHz USVs (Sadananda et al. 2012; Simola et al. 2012).
In the canonical conviction, dopaminergic reward system, originating from ventral tegmental area (VTA) is crucial for reward processing (Schultz et al. 1997; Dayan and Balleine 2002; Cohen et al. 2012; Lammel et al. 2012). The latest reports have shown that another structure—dorsal raphe might be equally treated as a key structure in reward processing and reward expectation (Luo et al. 2015, 2016; Li et al. 2016; Matthews et al. 2016; Qi et al. 2014). In mice with dopamine deficiency SSRIs (selective serotonin reuptake inhibitors) have produced robust conditioned place preference. This indicates that serotonin or serotonin-related mechanisms may mediate reward in the absence of dopamine (Hnasko et al. 2007).
It is well known that addictive drugs like morphine affect numerous neurochemical pathways, including those related to the reward system (Koob 1992). Morphine as MOR (µ-opioid) agonist has been shown to increase dopamine transmission via mechanisms of decreasing the release of GABA onto dopamine neurons in the VTA (Johnson and North 1992).
Acute administration of high doses of morphine (over 1.0 mg/kg) inhibited or did not evoke 50-kHz USVs (Nagai et al. 2004; Wright et al. 2012b; Simola et al. 2012; Hamed et al. 2012). Our previous studies showed that when morphine was administered for 14 days (once a day), an increase in the number of USVs episodes, recorded after drug injection, was observed on the 14th day as compared to the 1st and the 7th day (Hamed et al. 2012). Additionally, we have demonstrated that morphine-treated rats emitted much more ultrasonic vocalization in 50-kHz band in response to the context of morphine administration after withdrawal period compared to the same measurement before withdrawal period (Hamed et al. 2012). The neurochemical cause of this behavioral phenomenon still remains unknown. Moreover, several studies have shown that 50-kHz USVs are emitted during anticipation of the natural, pharmacological reward or even rewarding electrical brain stimulation (Burgdorf et al. 2000; Knutson et al. 1999; Opiol et al. 2015; Buck et al. 2014; Hamed et al. 2012). Simultaneously, existence of individual differences in the emission of 50-kHz USVs between rats further complicates the interpretation of behavioral data. Nevertheless, the fact that there are individual differences in the emission of the 50-kHz USVs allows us to explore neurochemical differences in structures related to reward processing, enabling us to explore biological basis of that phenomenon.
Taking into account that morphine pre-treated rats emitted much more context-induced 50-kHz USVs on the challenge day (after 14 day withdrawal period) and the aforementioned literature facts about pharmacological modifications of 50-kHz USVs we conducted non-parametric analysis between concentrations of neurochemical compounds in several brain structures (see “Materials and methods”) with the level of context-induced conditioned 50-kHz USVs. We hypothesized that the 50-kHz USVs emission could have separate neurochemical background in different behavioral paradigms as well as some common neurochemical mechanisms reflected in examined neurotransmitters correlations.
The aims of this study were to: (1) extract the most important neurotransmitters that are bound up with context-induced conditioned 50-kHz USVs related with pharmacological reward; (2) find biological basis of occurring inter-individual differences in context-induced conditioned 50-kHz USVs response; (3) create a model of all-to-all neurotransmitters correlations.
Materials and methods
Animals
Adult male Sprague–Dawley rats (n = 38; 180 ± 20 g) were used in the experiment. The animals were purchased from a licensed breeder (the Polish Academy of Science Medical Research Center, Warsaw, Poland). The animals were housed in standard laboratory conditions under 12 h:12 h light:dark cycles (lights on at 7 a.m.) at a constant temperature (21 ± 2 °C) and 70% humidity. The rats had free access to food and water. The experiments were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609 EEC). The Local Committee for Animal Care and Use of Warsaw Medical University approved all experimental procedures using animal subjects.
Experimental procedure
Groups of five animals were housed in acrylic cages (cage size: 54 cm × 34 cm × 21 cm) for 2 weeks. Morphine in dose 10.0 mg/kg (s.c.) was administered repeatedly to the experimental groups. Morphine and saline were administered in testing cages in a group of four animals for each cage. The testing room was significantly different than home cage room, both in the lighting conditions and in the arrangement of spatial cues that were constant throughout the whole experiment. The testing room was situated in a remote part of the laboratory. In Morph-D14 group, 13 injections were administered once a day. In the Morph-D28 group morphine was administered once a day for 14 days (10 mg/kg s.c). Saline solution was administered repeatedly (1.0 ml/kg) to control groups (Saline-D14 and) in the same manner as morphine in morphine treated rats. All animals were kept for 30 min in the testing box after each saline or morphine injection.
On day 14, Morph-D14 group and control Saline-D14 group were exposed to the context of drug administration, and the ultrasonic vocalizations were recorded for 20 min. Immediately after 20 min of USVs recording session rats from Morph-D14 and Saline-D14 groups were decapitated. On day 14, 30 min after last injection Morph-D28 and Saline-D28 group were left undisturbed (in their home cages), whilst they were subjected to 2-week withdrawal period. On day 28, the rats were re-exposed to the context of morphine/saline administration and ultrasonic vocalization was recorded for 20 min. Immediately after 20 min of USVs recording session rats from Morph-D28 and Saline-D28 groups were decapitated (Fig. 2). The USVs were recorded in a dark room with a dim red light [30 W bulb 1.5 m above the acrylic cage (size: 54 cm × 34 cm × 21 cm)]. The USVs response to the context was measured separately for each rat. To avoid the influence of scent marks from other animals on the behavior of subsequently tested rats, cages were cleaned with a 70% ethanol solution after each recorded session. Brain tissues were frozen in dry ice-cold isopentane, and stored at − 70 °C for neurochemical analysis.
Drugs
Morphine hydrochloride (Polfa, Poland) was dissolved in 0.9% isotonic saline. Morphine was administered in dose of 10.0 mg/kg (1.0 ml/kg; s.c.). Saline was used as a control vehicle (1 ml/kg).
Apparatus and USVs recordings
All subtypes of 50 kHz rat calls were recorded using an UltraSoundGate Condenser Microphone CM16 (Avisoft Bioacoustics, Berlin, Germany) that was positioned 25–30 cm above the floor of the cage. This microphone was sensitive to frequencies of 15–180 kHz with a flat frequency response (± 6 dB) between 25 and 140 kHz. It was connected to an amplifier (custom-made) that had the following parameters: a voltage gain of 16 V/V (12 dB), a frequency response of ± 0.1 dB, a range of 30 Hz to 120 kHz, and an input impedance of 600Ω. The signal was then transferred through a 120 kHz anti-aliasing filter (custom-made). The filtered sounds were sent to a PCI-703-16A data acquisition board (Eagle Technology, USA). This board was a 14-bit 400-kHz analogue input and analogue output board for PCI-based systems. The recorded data were processed using the RAT-REC PRO 5.0 software (custom-made). The signals were processed through a fast Fourier-transformation (1024, Hamming window) and displayed as color spectrograms. Each signal was manually marked with the section label included in the automated parameter measurement. Various parameters were determined automatically, including the number of USV calls, the total calling time (s), the mean call length (s), the frequency bandwidth (kHz), the number of gaps, the mean gap length (s), and the mean peak frequency (kHz). Only the number of calls is included as a main parameter in the results presented here. Taking into account that dopaminergic system plays role in the processing of both appetitive and aversive states (Bromberg-Martin et al. 2010; Zweifel et al. 2011; Lammel et al. 2011a, b), we have analyzed FFT spectrograms in whole recorded frequency spectrum (10–130 kHz) to evaluate occurrence not only “50-kHz appetitive”, but also “22-kHz aversive” calls. Detailed analysis of the FFT spectrograms showed the absence of 22-kHz (alarm calls) in presented model.
HPLC analysis of monoamines
Frozen brains were cut into slices (− 20 °C) in a cryostat. The following structures were dissected: prefrontal cortex (3.7–3.2 mm anterior to the bregma); nucleus accumbens (NAcc) and caudate putamen (CPu) (1.70–1.0 mm anterior to the bregma); hippocampus and amygdala (− 2.80 to − 3.60 mm posterior to the bregma); and ventral tegmental area (− 5.80 to − 6.30 mm posterior to the bregma) based on the rat brain atlas of Paxinos and Watson (1998). Tissue samples were weighed and homogenized for 30 s in 15 volumes of ice-cold 0.2 M perchloric acid, which contained dihydroxybenzylamine as an internal standard. The homogenates were then centrifuged at 26,900g for 8 min at 4 °C. The supernatants were then filtered through 0.45-μm pore filters and stored at − 70 °C until analyzed for noradrenaline (NA), 3-methoxy-4-hydroxyphenylglycol (MHPG), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 3-methoxytyramine (3-MT), serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) using HPLC, as described by Kaneda et al. (1986), with minor modifications (Szyndler et al. 2010). The concentrations of NA, MHPG, DA, DOPAC, 3-MT, HVA, 5-HT and 5-HIAA were calculated as ng/g of brain tissue. Additionally, to approximate their turnover, the following concentration ratios were computed: 3-MT to DA, DOPAC to DA, HVA to DA, 5-HIAA to 5-HT and MHPG to NA.
HPLC analysis of amino acids
The brain levels of the amino acids were determined using HPLC method, coupled to an electrochemical detection, as described previously (Szyndler et al. 2008). Briefly, the compounds were eluted isocratically with the mobile phase delivered at 0.7 ml/min, using a Shimadzu Class LC-10ADvp pump. An electrochemical detector with a flow-through cell (Intro-Antec Leyden) linked to the Shimadzu Class VP Integrator SCL-10 Avp was used. A high-density glass carbon-working electrode (Antec) was used at + 0.85 V. A Rheodyne injection valve with 20-µl sample loops was used to manually inject the samples. The preparation of the mobile phase and the derivatizing agents was based on the slightly modified method of Rowley et al. (1995; Szyndler et al. 2008). The concentrations of GABA, alanine, taurine, glutamine and glutamate were calculated in µmol/g of tissue. Similarly as in case of monoamines, ratios of the glutamate concentration to both glutamine and GABA were also computed and investigated.
Statistics
All levels of compounds across brain structures and the aforementioned level ratios formed a set of 130 descriptors of the brain state. One should note, however, that the introduction of ratios has created certain artificial correlations, disallowing descriptor independence assumptions.
According to a Shapiro–Wilk test, distributions of most (53%) of the predictors analyzed in this study are not normally distributed, hence non-parametric statistical approaches were predominantly used.
Relations between numerical variables were assessed using the Spearman correlation, as it is not only robust to non-normality, but can be effectively assessed in terms of significance assuming independence as a null hypothesis, even exactly for small sample sizes.
On the other hand, relations between numerical and binary variables were assessed using the Mann–Whitney–Wilcoxon test.
Still, these tests do not consider possible multivariate associations; to this end, the Boruta machine learning method was employed (Kursa and Rudnicki 2010).
It works by iterative fitting of the Random Forest (Breiman 2001) model to the data, and extracting variables that are significantly more useful for that purpose than shadows, design injected into the dataset. When not specified otherwise, Holm–Bonferroni method was used to correct for multiple comparisons, and the significance level was set at p = 0.05. All computations were performed using R 3.4.1 (R Core Team 2017) with the Boruta 5.2.0 (Kursa and Rudnicki 2010), pspearman 0.3–0 (Savicky 2014) and ranger 0.8.0 packages (Wright and Ziegler 2017).
Results
The effects of morphine administration and withdrawal period on context-induced conditioned 50-kHz USVs
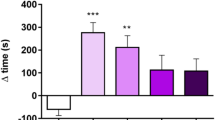
The effects of morphine and withdrawal period on context-induced conditioned 50-kHz USVs count has been analyzed with a series of 6 Mann–Whitney–Wilcoxon tests, and corrected for multiple comparisons. The only non-significant difference between USV counts (number of 50-kHz USVs episodes) in experimental groups was observed between saline and morphine groups on day 14. Between day 14 and day 28, the USV count has increased both in saline (p = 0.03) and morphine (p = 0.002) groups, while on day 28 the morphine-treated rats have vocalized more frequently than saline-treated (p = 0.02) (Figs. 3, 4).
The correlations between USV counts and neurotransmitter levels
Next, the links between USV count and levels or level ratios of the analyzed compounds in across brain structures were assessed. To this end, Spearman correlation test was used, also allowing for extracting the sign of correlation. Additionally, the Boruta machine learning method was used to investigate potential multivariate interactions. Such analysis was performed for all rats, as well as in context of morphine- and saline-treated group.
The strongest, significant correlations with USV count were: with 5-HT level in amygdala, hippocampus (for all rats) and medial prefrontal cortex (for morphine group), as well as with 5-HIAA in hippocampus (for all rats). Furthermore, we found USV count to be significantly correlated with the Glu/Gln ratio in nucleus accumbens for morphine group. The raw data behind this interactions are shown on Fig. 4. All these results were also confirmed by the Boruta analysis, along with a number of lesser interaction, collected in Table 1.
The effect of the re-exposure to the morphine-paired context on neurotransmitters levels
Similar analysis was performed to analyze which compounds can be linked with context-induced conditioned response. This is a classification problem, hence Mann–Whitney–Wilcoxon test was used instead of Spearman correlation. The Boruta method can handle both classification and regression problems, so the auxiliary analysis involving this approach was performed exactly in the same way as in the previous case. Again, contexts of all rats, morphine and saline groups were investigated separately. Especially, we found positive associations between context conditioning and serotonin levels in amygdala, hippocampus and mPFC, as well as with Glu/Gln ratio in nucleus accumbens, both for all rats and only morphine group. The other interesting effects are: increase of Glu/GABA ratio in VTA and in amygdala for all rats, increased glutamate in amygdala for all rats and only for morphine group, decrease of GABA in VTA and mPFC for all rats and only for morphine group. The said analysis yield numerous significant results, which are summarized in Table 2.
The effects within neurotransmitter levels
Based on the results highlighted above and theoretical considerations, four substantial descriptors were selected: 5-HT and glutamate levels in amygdala, the ratio of glutamate and glutamine levels in nucleus accumbens, finally the levels of glutamate in hippocampus. For these, sets of other descriptors that significantly correlated with each of them were identified. To this end, Spearman correlation-based methodology identical to previously used for associations with the USV count was applied. All significant correlations with the serotonin level in amygdala are presented in Fig. 5; they include positive correlations with 5-HT levels in other structures (Nacc, Cpu and hippocampus), serotonin metabolite 5-HIAA in amygdala, as well as with the levels of glutamate in both amygdala and hippocampus. On the other hand, negative correlations were observed with the levels of taurine, alanine and GABA in medial prefrontal cortex. Figure 6 contains all the significant correlations with the level of glutamate in amygdala; similarly, it was correlated positively with levels of 5-HT and glutamine, and negatively with levels of alanine and taurine in mPFC. A positive correlation with the level of taurine in amygdala was also detected. The correlations of Glu/Gln ratio in nucleus accumbens are collected in Fig. 7. This set contains positive correlations with the same ratio in other structures: amygdala, Cpu, hippocampus and VTA, as well as negative with glutamine in mPFC and Cpu; also a positive correlation with serotonin and 5-HIAA in amygdala was identified. An interesting finding is a correlation between Glu/Gln in Nacc and MHPG/NA ratio in Cpu. Finally, all significant correlations of Glu levels in hippocampus are collected in Fig. 8; they include positive correlations with: MHPG/NA ratio in Cpu; GABA, glutamine, taurine level and Glu/GABA ratio in hippocampus; glutamate level in VTA, serotonin concentration in amygdala.
Intra-neurotransmitter level correlation structure
Finally, a global, all with all correlation scan was performed. To avoid artificial correlations, the ratios were discarded for this analysis. It was also performed using Spearman correlation test, although the Benjami Hochberg’s multiple comparison correction (FDR) (Benjamini and Hochberg 1995) was used. The results of conducted analysis were compiled into a graph, nodes of which correspond to compound levels across structures, while edges to correlations significant at p = 0.03 level. The graph was manually laid out to expose relevant coherent structures, and presented in Fig. 9.
Graph of significant correlations between compound levels. Node shape denotes significant relation with post-withdrawal conditioned response to the context, respectively: triangle up—level elevated in post-withdrawal conditioned response to the context, triangle down—lowered, square—interaction identified exclusively by machine learning. Node outline denotes significant relation with the USV episode count, respectively: black outline—positive correlation, grey outline—interaction identified exclusively by machine learning. Edge with corresponds to the correlation strength, while its colour to the correlation sign: grey denotes positive, while fuchsia negative. Node colours denote brain structure
Discussion
The data collected here confirms our previous behavioral results that re-exposure to the context of morphine administration after the withdrawal period (day 28) increases the level of 50-kHz USVs episodes, compared to day 14 (Fig. 3) (Hamed et al. 2012). Context-induced 50-kHz USVs emission is variable among rats, what indicates individual differences in context conditioned response. This kind of gradation allows us to analyze individual differences in brain neurotransmission and understand the basis of occurring variations in response to the context.
Interestingly, in control saline group an increase of 50-kHz USVs was also observed on day 28 compared to day 14. One of the reasons might be that both morphine and saline were administered in testing cages in a group of four animals for each cage (see “Materials and methods”). This explanation could suggest that the context conditioned response was enhanced by the natural social contact associated with a different, uncommon environment. Considering rewarding effects of morphine reflected by 50-kHz USVs emission reported in our previous studies (Hamed et al. 2012), we can be sure that emitted sounds reflect context-induced conditioned response related to morphine rewarding effects. This kind of behavioral 50-kHz USVs response was reported in studies with natural, pharmacological reward or even with rewarding electrical brain stimulation (Knutson et al. 1999; Burgdorf et al. 2000; Opiol et al. 2015; Buck et al. 2014). The level of emitted 50-kHz USVs was over ten times higher in morphine-treated group than in saline group and only three of saline control animals produced more than hundred USVs episodes (Figs. 3, 4). The starting point of our correlations analysis was the level of context conditioned response reflected by 50-kHz USVs emission. All further consecutive analysis were conducted in the same way in the morphine and saline-treated animals (Figs. 4, 5, 6, 7, 8, 9; Tables 1, 2).
Nevertheless, the fact that emission of 50-kHz USVs is a context-induced conditioned response, it is interesting why re-exposure to the drug-paired context did not evoke such sounds on day 14 in the same manner as on day 28 (after 14 day-withdrawal period; Figs. 3, 4). This time-dependent differentiation effect of observed behavior may be explained by the phenomenon defined as “incubation of drug craving”. It was demonstrated in humans that craving may be triggered by drug-associated cues and may increase progressively during the early days of withdrawal (Gawin and Kleber 1986; Abrams et al. 1988; Drummond et al. 1990; George et al. 2001; Drummond 2000; Li et al. 2012). In rodents, incubation of craving has been demonstrated both in drug-treated animals (Grimm et al. 2001; Shalev et al. 2001; Shepard et al. 2004; Abdolahi et al. 2010) as well as in non-drug reward experiments (Grimm et al. 2002). Incubation of craving involves neuroadaptations in reward and motivation-related structures (Conrad et al. 2008; Sesack and Grace 2010; Wolf and Tseng 2012; Lee et al. 2013; Purgianto et al. 2013; Ma et al. 2014; Pascoli et al. 2014; Li et al. 2015; Wolf 2016). It is well known that nucleus accumbens is key structure of the limbic system that is highly related to reward processing (Berridge 2007; Ikemoto 2007) and 50-kHz USVs emission (Thompson et al. 2006; Brudzynski 2013a, b; Hamed et al. 2016).
The present study shows that 50-kHz USVs emission is associated with increased serotonin concentrations in amygdala, hippocampus and mPFC and also with elevated Glu/Gln ratio in nucleus accumbens (Fig. 4; Table 1). Moreover, concentration of serotonin in amygdala is directly proportional with the level of serotonin in nucleus accumbens and in hippocampus as well as with concentration of glutamate in amygdala and hippocampus (Fig. 5).
It was found that central amygdala (CeA) plays a critical role in incubation of drug and non-drug reward craving (Uejima et al. 2007; Li et al. 2008, 2015). Inhibition of CeA neuronal activity reduced incubation of nicotine, cocaine, methamphetamine and sucrose craving (Funk et al. 2016; Lu et al. 2005, 2007; Uejima et al. 2007; Li et al. 2015). Human fMRI studies report that amygdala is the key structure in the generation of cue-elicited opioid craving (Langleben et al. 2008, 2014; Mei et al. 2010; Li et al. 2012; Murphy et al. 2017). It was found that GABA-ergic inhibition of neuronal activity leads to reduction of behavioral expression of craving (Li et al. 2015). It was also demonstrated that ethanol withdrawal reduced GABA levels in medial prefrontal cortex and enhanced glutamate and glutamine levels in NAcc (Hinton et al. 2012). In our study, we found that GABA as well as alanine and taurine concentrations in mPFC are inversely proportional to the concentration of serotonin in the amygdala (Fig. 5) and the concentration of GABA in mPFC was decreased after withdrawal period. These data indicate that reduction of GABA-ergic inhibition in mPFC may be one of the initiating factors of drug-seeking behavior and expression of craving reflected by 50-kHz USVs. We have also demonstrated for the first time that Glu/GABA ratio in VTA and amygdala was elevated after re-exposure to the drug-paired context on the day 28 compared to day 14 (Table 2).
It was demonstrated in optogenetic studies that silent synapse-based reorganization of the amygdala-to-accumbens projections plays key role in stability of cocaine craving and relapse after withdrawal period (Lee et al. 2013). Furthermore, it was found that mechanisms related to the action of MMP-9 (an important controller of the synaptic plasticity of excitatory synapses) in central amygdala are crucial in generating a motivation for reward seeking (Stefaniuk et al. 2017). An elevated glutamate concentration in amygdala which correlates with increased serotonin in this structure (Fig. 6), parallels an experiment in which the reduction of serotonergic neurotransmission in amygdala promoted hyperexcitability of this structure by enhancing glutamatergic neurotransmission, in consequence increasing fear related behaviors (Tran et al. 2013). It might further indicate that increased concentration of serotonin in this structure presented in our study, prevents or switches over the arousal from fear expression to appetitive arousal related to drug-seeking behavior expressed by 50-kHz emission. Further investigations of mentioned neurochemicals co-existence in the amygdala have to be performed to address this hypothesis. Moreover, ex vivo electrophysiological recordings with optogenetic methods and pharmacological analysis revealed the existence of the 5-HT and glutamate co-transmission in basal amygdala neurons (Sengupta et al. 2017).
The very nature of the conditioned place preference is attributed to activation of the reward system and its association with information on the surrounding space during training sessions. This information in the hippocampus is represented by a subset of spatially tuned neurons called place cells (Hollup et al. 2001). The hippocampus and its inputs have been implicated in memory formation, including reward-related memory (Scoville and Milner 1957; Lisman and Grace 2005; Hernández-Rabaza et al. 2008; Bunzeck et al. 2011). Furthermore, it was demonstrated that a projection from area CA3 of dorsal hippocampus to ventral tegmental area (VTA) mediated relations between context and reward (Luo et al. 2011). Ntamati and Luscher identified and characterized a projection from VTA that releases both glutamate and GABA near the granule cells of hippocampal DG (Ntamati and Lüscher 2016).
We found that the level of serotonin in amygdala is directly proportional to the concentration of glutamate in amygdala and hippocampus as well as to the level of serotonin in nucleus accumbens and in the hippocampus (Fig. 5). Additionally, all-to-all analysis indicate that concentration of glutamate in hippocampus is directly proportional to glutamate level in VTA and GABA concentration in the hippocampus (Figs. 8, 9).
There are potent structural interconnectivities and functional relationships between nucleus accumbens (Nacc), amygdala and hippocampus. Nucleus accumbens integrates cortical and limbic glutamatergic inputs arising from basolateral amygdala (BLA), hippocampal ventral subiculum (vSub) and prefrontal cortex (PFC) (Phillipson and Griffiths 1985; Groenewegen et al. 1987; McDonald 1991; Shinonaga et al. 1994; Johnson et al. 1994; O’Donnell and Grace 1995; Petrovich et al. 1996; Friedman et al. 2002; French and Totterdell 2003; Sesack and Grace 2010; Gill and Grace 2011; Britt et al. 2012).
It is well known that the amygdala is a structure involved in expression of the emotion and in learned emotional behaviors (LeDoux 2000). Previous studies have established that the BLA is involved in affective response and the vSub in context dependency (Gill and Grace 2011). Optogenetic studies revealed that selective activation of BLA, but not mPFC, glutamatergic inputs to the NAcc promotes motivated behavioral responding (Stuber et al. 2011). On the other hand, photostimulation of each of the different afferent pathways (vHipp, mPFC, BLA axons) to the NAcc reinforced instrumental behavior (Britt et al. 2012). Britt and colleagues suggested that the specific pathway releasing glutamate is not as important as the amount of glutamate that is released (Britt et al. 2012). Anatomical and functional interconnectivity of the amygdala and hippocampus has been demonstrated in a number of studies (Mello et al. 1992a, b; Maren and Fanselow 1995; Ikegaya et al. 1994, 1996a, b; Akirav and Richter-Levin 1999; Nakao et al. 2004).
We did not detect correlative association of glutamate level between hippocampus and amygdala, but we found strong relation between glutamate concentration in hippocampus and the serotonin level in amygdala (Figs. 8, 9). Considering the fact that the hippocampus is strictly involved in spatial memory formation, we can suppose that, after or during activation of hippocampus-VTA loop the hippocampus sends glutamatergic signal to the amygdala to amplify the serotonergic signaling. Then, 5-HT augmentation may initiate or support the glutamatergic signaling in amygdala. These processes might have a strong impact on the 50-kHz USVs expression of emotions reflected in neurochemical changes in Glu/Gln ratio in Nacc. Understanding the sequence of activation of the neurochemical signaling between interacting structures requires further research using electrophysiological, optogenetic or voltammetric methodology.
Obtained neurochemical data confirms the contribution of amygdala, nucleus accumbens and hippocampus to spatial memory formation and memory processing bound up with enhanced emotional states (arousal) triggered by an appetitive conditioned context (Hall et al. 2001; Ito et al. 2006, 2008). Taking into account the strong serotoninergic response simultaneously in amygdala, nucleus accumbens, mPFC and hippocampus in processing context conditioned response, based on literature reports, it is reasonable to assume that the structure responsible for this increased levels of 5-HT was dorsal raphe. This structure represents one of the most sensitive reward sites in the brain (Yi Li et al. 2016; Luo et al. 2015, 2016; Matthews et al. 2016; Qi et al. 2014). It has been shown that the nucleus accumbens receives serotonin and non-serotonin inputs from dorsal raphe nucleus (Van Bockstaele and Pickel 1993; Brown and Molliver 2000). Unfortunately, because we did not expect such strong serotonin response in case of 50-kHz USVs emission, we did not analyze concentrations of the neurochemical compounds in dorsal raphe.
Rewards such as food, sucrose, social interaction or sex rapidly activate serotonin neurons in dorsal raphe (Yi Li et al. 2016). It was also demonstrated that dorsal raphe neurons encode reward via serotonin and glutamate (Liu et al. 2014). It is well known that SSRIs affecting the serotonergic system have been used extensively in the treatment of psychiatric disorders over past 20 years. In the studies using microdialysis methods, acute treatment with citalopram increased extracellular serotonin concentration in the central amygdala to 175% (Bosker et al. 2001) of basal level and in the ventral hippocampus citalopram increased serotonin to 325% of the basal level (Cremers et al. 2000). It is also interesting that studies on dopamine-deficient mice have demonstrated that dopamine is not required for morphine-induced reward as measured by conditioned place preference (Hnasko et al. 2005), which indicates that dopaminergic mesolimbic system is not crucial for reward processing and acquisition. Hnasko et al. (2007) demonstrated that in mice with dopamine deficiency the fluoxetine at dose of 5.0 mg/kg produced robust conditioned place preference. This indicate that serotonin may mediate reward in the absence of dopamine (Hnasko et al. 2007).
It is well known that glutamate is the primary excitatory neurotransmitter in the nervous system (Sladeczek et al. 1985). Biochemical studies demonstrate that astrocytic glutamine plays a crucial role in sustaining excitatory neurotransmission (Hertz 1979; Tani et al. 2014). Given the presence of the physiological glutamate–glutamine cycle (Hertz 1979; Scofield and Kalivas 2014; Tani et al. 2014) we have analyzed the correlation of behavioral and biochemical data with Glu/Gln ratio in all examined structures. Analysis of another neurotransmitter/metabolite ratios were also done (see “Statistics”).
We have shown for the first time that the number of USVs episodes strongly correlates with Glu/Gln ratio in nucleus accumbens (Fig. 4) and the increased Glu/Gln ratio in nucleus accumbens has strong associations with Glu/Gln ratio simultaneously in VTA, amygdala, CPu and hippocampus (Fig. 7). This parameter also positively correlates with concentrations of serotonin and its metabolite 5-HIAA in amygdala as well as with MHPG/NA ratio in CPu (Fig. 7). Increased levels of Glu/Gln ratio in amygdala presented in our study is interesting in light of data from electrochemical studies, which indicate that glutamate concentration in basolateral amygdala is transiently elevated by reward-predictive stimuli (Malvaez et al. 2015). Moreover, previous studies showed that blockade of the glia selective glutamate reuptake in the amygdala and in VTA induced depressive-like effects, including anhedonic symptoms manifested in reduced social interactions and reduced sensitivity to reward (Herberg and Rose 1990; Lee et al. 2007; Bechtholt-Gompf et al. 2010; John et al. 2012). It was also demonstrated that dentate gyrus of the hippocampus was the main structure responsible for this effect and impaired spatial memory was related to observed anhedonic symptoms (Bechtholt-Gompf et al. 2010). Furthermore, it was reported that decreased levels of astrocytic glutamate transporter occurred in animal models of depression (Zink et al. 2010). It is worth mentioning that the most commonly used anti-depressive drug fluoxetine (selective inhibitor of the serotoninergic transporter) induced astrocytic glutamate transporter expression in hippocampus, amygdala and retrosplenial granular cortex (Zink et al. 2011).
Thus, the relationship between the emission of 50-kHz USVs and the neurochemical changes that occur after re-exposure to morphine-paired context, indicates strong serotoninergic response in amygdala, hippocampus and mPFC enhanced with increased glutamatergic activity in nucleus accumbens. Most of the studies considering ‘incubation craving’ were carried out with more than 2 weeks of withdrawal period. Nevertheless, the time-dependent differentiation of behavioral response presented in our study indicate that context-induced 50-kHz USVs emission might be a new tool for reflecting individual differences in incubation of craving. Presented analysis indicates a strong correlation between serotonergic and glutamatergic systems in context-induced conditioned response. The strength of this co-transmission correlates with the number of 50-kHz USVs emitted in response to the reward context.
Limitation of the study
The limitation of the procedure is the lack of control in another environment that was not paired with morphine conditioning. This kind of neutral environment control would exclude chance that 50-kHz USVs might be reflection of incubation of craving triggered without drug-associated spatial cues and the 50-kHz USVs emission might be permanent after 14 days of withdrawal period. Nevertheless, context-induced conditioning USVs was not observed in the first seconds of testing session and these sounds were intensifying with the time spent in the testing cage, probably after acquisition spatial cues.
Another limitation of our study is that the analysis of neurotransmitters concentration was performed post mortem, so there was a time lag between measurement of USV and measurement of neurochemical changes in the brain. However, animals were decapitated immediately after testing session and brain tissue of each animal was isolated strictly after that. It is well known that the advantage of the in vivo technique (voltammetry or microdialysis) is that it enables tracking of changes in the extracellular concentrations of neurotransmitters over time; however, monitoring is generally limited to changes in a single structure. The ex vivo method enables the analysis of not only the extracellular concentration but also the total concentration of neurotransmitters and their metabolites in several brain structures. According to our knowledge, there is no technological possibility to analyze concentration of many neurotransmitters and their metabolites in several structures in vivo in freely moving rats.
References
Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ (2010) Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci 31:733–741
Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI (1988) Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther 26:225–233
Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T (2009) Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res 197(1):205–209
Akirav I, Richter-Levin G (1999) Priming stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. Neurosci Lett 270:83–86
Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA Jr, Ongür D, Cohen BM (2010) Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 35(10):2049–2059
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57(1):289–300
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikström HV, den Boer JA (2001) Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem 76(6):1645–1653
Breiman L (2001) Random forests. Mach Learn 45:5–32
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76:790–803
Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68(5):815–834
Brown P, Molliver ME (2000) Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J Neurosci 20(5):1952–1963
Brudzynski SM (2013a) Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23:310–317
Brudzynski SM (2013b) Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23(3):310–317
Brudzynski SM, Pniak A (2002) Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol 116(1):73–82
Brudzynski SM, Silkstone M, Komadoski M, Scullion K, Duffus S, Burgdorf J, Kroes RA, Moskal JR, Panksepp J (2011) Effects of intraaccumbens amphetamine on production of 50 kHz vocalizations in three lines of selectively bred Long–Evans rats. Behav Brain Res 217(1):32–40
Buck CL, Vendruscolo LF, Koob GF, George O (2014) Dopamine D1 and mu-opioid receptor antagonism blocks anticipatory 50 kHz ultrasonic vocalizations induced by palatable food cues in Wistar rats. Psychopharmacology 231:929–937
Bunzeck N, Guitart-Masip M, Dolan RJ, Düzel E (2011) Contextual novelty modulates the neural dynamics of reward anticipation. J Neurosci 31(36):12816–12822
Burgdorf J, Knutson B, Panksepp J (2000) Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci 114(2):320–327
Burgdorf J, Knutson B, Panksepp J, Ikemoto S (2001) Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalization in rats. Behav Neurosci 115:940–944
Burgdorf J, Wood P, Kroes R, Moskal J, Panksepp J (2007) Neurobiology of 50 kHz ultrasonic vocalization in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res 182:274–283
Burgdorf J, Colechio EM, Stanton P, Panksepp J (2017) Positive emotional learning induces resilience to depression: a role for NMDA receptor-mediated synaptic plasticity. Curr Neuropharmacol 15(1):3–10
Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482:85–88
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121
Costa G, Morelli M, Simola N (2015) Involvement of glutamate NMDA receptors in the acute, long-term, and conditioned effects of amphetamine on rat 50 kHz ultrasonic vocalizations. Int J Neuropsychopharmacol 18(11):pyv057
Cremers TI, de Boer P, Liao Y, Bosker FJ, den Boer JA, Westerink BH, Wikström HV (2000) Augmentation with a 5-HT(1A), but not a 5-HT(1B) receptor antagonist critically depends on the dose of citalopram. Eur J Pharmacol 397(1):63–74
Dayan P, Balleine BW (2002) Reward, motivation, and reinforcement learning. Neuron 36:285–298
Drummond DC (2000) What does cue-reactivity have to offer clinical research? Addiction 95:129–144
Drummond DC, Cooper T, Glautier SP (1990) Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict 85:725–743
French SJ, Totterdell S (2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119(1):19–31
Friedman DP, Aggleton JP, Saunders RC (2002) Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the macaque brain. J Comp Neurol 450:345–365
Fu XW, Brudzynski SM (1994) High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacol Biochem Behav 49(4):835–841
Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD (2016) Role of central amygdala neuronal ensembles in incubation of nicotine. Craving J Neurosci 36(33):8612–8623
Gawin FH, Kleber HD (1986) Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43:107–113
George MS, Anton RF, Bloomer C, Teneback Ch, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ (2001) Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 58:345–352
Gill KM, Grace AA (2011) Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol 14(10):1301–1314
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412:141–142
Grimm JW, Shaham Y, Hope BT (2002) Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13:379–388
Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP (1987) Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23(1):103–120
Hall J, Thomas KL, Everitt BJ (2001) Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci 21:2186–2193
Hamed A, Jaroszewski T, Maciejak P, Szyndler J, Lehner M, Kamecka I, Plaznik A (2009) The effects of buspirone and diazepam on aversive context- and social isolation-induced ultrasonic vocalization. Physiol Behav 98:474–480
Hamed A, Taracha E, Szyndler J, Krząścik P, Lehner M, Maciejak P, Skórzewska A, Płaźnik A (2012) The effects of morphine and morphine conditioned context on 50 kHz ultrasonic vocalisation in rats. Behav Brain Res 229(2):447–450
Hamed A, Szyndler J, Taracha E, Turzyńska D, Sobolewska A, Lehner M, Krząścik P, Daszczuk P (2015) κ-opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations. Psychopharmacology 232(11):1941–1955
Hamed A, Daszczuk P, Kursa MB, Turzyńska D, Sobolewska A, Lehner M, Boguszewski PM, Szyndler J (2016) Non-parametric analysis of neurochemical effects and Arc expression in amphetamine-induced 50-kHz ultrasonic vocalization. Behav Brain Res 312:174–185
Herberg LJ, Rose IC (1990) Excitatory amino acid pathways in brain-stimulation reward. Behav Brain Res 20(3):230–239
Hernández-Rabaza V, Hontecillas-Prieto L, Velázquez-Sánchez C, Ferragud A, Pérez-Villaba A, Arcusa A, Barcia JA, Trejo JL, Canales JJ (2008) The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem 90:553–559
Hertz L (1979) Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol 13(3):277–323
Hinton DJ, Lee MR, Jacobson TL, Mishra PK, Frye MA, Mrazek DA, Macura SI, Doo-Sup C (2012) Ethanol withdrawal-induced brain metabolites and the pharmacological effects of acamprosate in mice lacking ENT1. Neuropharmacology 62(8):2480–2488
Hnasko TS, Sotak BN, Palmiter RD (2005) Morphine reward in dopamine-deficient mice. Nature 438(7069):854–857
Hnasko TS, Sotak BN, Palmiter RD (2007) Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci 27(46):12484–12488
Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI (2001) Place fields of rat hippocampal pyramidal cells and spatial learning in the watermaze. Eur J Neurosci 13(6):1197–1208
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Ikegaya Y, Saito H, Abe K (1994) Attenuated hippocampal longterm potentiation in basolateral amygdala-lesioned rats. Brain Res 656:157–164
Ikegaya Y, Saito H, Abe K (1996a) Dentate gyrus field potentials evoked by stimulation of the basolateral amygdaloid nucleus in anesthetized rats. Brain Res 718:53–60
Ikegaya Y, Saito H, Abe K (1996b) The basomedial and basolateral amygdaloid nuclei contribute to the induction of long-term potentiation in the dentate gyrus in vivo. Eur J Neurosci 8:1833–1839
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev 56:27–78
Ito R, Robbins TW, McNaughton BL, Everitt BJ (2006) Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J Neurosci 23(11):3071–3080
Ito R, Robbins TW, Pennartz CM, Everitt BJ (2008) Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci 28(27):6950–6959
John CS, Smith KL, Van’t Veer A, Gompf HS, Carlezon WA Jr, Cohen BM, Öngür D, Bechtholt-Gompf AJ (2012) Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology 37(11):2467–2475
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12(2):483–488
Johnson LR, Aylward RL, Hussain Z, Totterdell S (1994) Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience 61(4):851–865
Kaneda N, Asano M, Nagatsu T (1986) Simple method for simultaneous determination of acetylcholine, choline, noradrenaline, dopamine and serotonin in brain tissue by high-performance liquid chromatography with electrochemical detection. J Chromatogr 36:211–218
Knutson B, Burgdorf J, Panksepp J (1998) Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol 112:65–73
Knutson B, Burgdorf J, Panksepp J (1999) High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav 66(4):639–643
Koob GF (1992) Drug of abuse: anatomy, pharmacology and function of reward pathways. Trands Pharmacol Sci 13:177–184
Kursa MB, Rudnicki WR (2010) Feature selection with the Boruta Package. J Stat Softw 36(11):1–13. http://www.jstatsoft.org/v36/i11/
Lammel S, Ion DI, Roeper J, Malenka RC (2011a) Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70:855–862
Lammel S, Ion DI, Roeper J, Malenka RC (2011b) Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70(5):855–862
Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature 491:212–217
Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR (2008) Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry 165:390–394
Langleben DD, Ruparel K, Elman I, Loughead JW, Busch EL, Cornish J, Lynch KG, Nuwayser ES, Childress AR, O’Brien CP (2014) Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol 19:262–271
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Lee Y, Gaskins D, Anand A, Shekhar A (2007) Glia mechanisms in mood regulation: a novel model of mood disorders. Psychopharmacology 191(1):55–65
Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schluter OM, Dong Y (2013) Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 16:1644–1651
Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L (2008) Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28:13248–13257
Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, Wu N, Chang H, Zheng Y, Qin W, Zhao L, Yuan K, Liu J, Wang W, Tian J (2012) Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res 1469:63–72
Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y (2015) The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology 40(5):1297–1306
Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M (2016) Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7:10503
Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M (2016) Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7:10503
Lisman JE, Grace AA (2005) The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46(5):703–713
Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, Bao J, Kim JY, Chen ZF, El Mestikawy S, Luo M (2014) Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81(6):1360–1374
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005) Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8(2):212–219
Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y (2007) Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry 61(5):591–598
Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G (2011) Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 15(6040):353–357
Luo M, Zhou J, Liu Z (2015) Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem 22(9):452–460
Luo M, Li Y, Zhong W (2016) Do dorsal raphe 5-HT neurons encode “beneficialness”? Neurobiol Learn Mem 135:40–49
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467
Malvaez M, Greenfield VY, Wang AS, Yorita AM, Feng L, Linker KE, Monbouquette HG, Wassum KM (2015) Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci Rep 5:12511
Maren S, Fanselow MS (1995) Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci 15(11):7548–7564
Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, Wildes CP, Ungless MA, Tye KM (2016) Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164(4):617–631
McDonald AJ (1991) Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 44(1):15–33
Mei W, Zhang JX, Xiao Z (2010) Acute effects of sublingual buprenorphine on brain responses to heroin-related cues in early-abstinent heroin addicts: an uncontrolled trial. Neuroscience 170:808–815
Mello LE, Tan AM, Finch DM (1992a) GABAergic synaptic transmission in projections from the basal forebrain and hippocampal formation to the amygdala: an in vivo iontophoretic study. Brain Res 31:41–48. 88
Mello LE, Tan AM, Finch DM (1992b) Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: an in vivo extra- and intracellular electrophysiological study. Brain Res 31:24–40
Murphy A, Lubman DI, McKie S, Bijral PS, Peters LA, Faiz Q, Holmes SE, Anderson IM, Deakin B, Elliott R (2017) Time-dependent neuronal changes associated with craving in opioid dependence: an fMRI study. Addict Biol. https://doi.org/10.1111/adb.12554
Nagai T, Yamada K, Yoshimura M, Ishikawa K, Miyamoto Y, Hashimoto K, Noda Y, Nitta A, Nabeshima T (2004) The tissue plasminogen activator-plasmin system participates in the rewarding effect of morphine by regulating dopamine release. Proc Natl Acad Sci USA 101(10):3650–3655
Nakao K, Matsuyama K, Matsuki N, Ikegaya Y (2004) Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci USA 101(39):14270–14275
Ntamati NR, Lüscher C (2016) VTA projection neurons releasing GABA and glutamate in the dentate gyrus. eNeuro 3(4)
O’Donnell P, Grace AA (1995) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15(5 Pt 1):3622–3639
Opiol H, Pavlovski I, Michalik M, Mistlberger RE (2015) Ultrasonic vocalizations in rats anticipating circadian feeding schedules. Behav Brain Res 284:42–50
Panksepp J, Burgdorf J (2003) “Laughing” rats and the evolutionary antecedents of human joy? Physiol Behav 79(3):533–547
Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C (2014) Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509(7501):459–464
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Petrovich GD, Risold PY, Swanson LW (1996) Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 374(3):387–420
Phillipson OT, Griffiths AC (1985) The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16(2):275–296
Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME (2013) Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology 38(9):1789–1797
Qi J, Zhang S, Wang HL, Wang H, de Jesus Aceves Buendia J, Hoffman AF, Lupica CR, Seal RP, Morales M (2014) A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat Commun 5:5390
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rowley HL, Martin KF, Marsden CA (1995) Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods 57:93–99
Sadananda M, Natusch C, Karrenbauer B, Schwarting RK (2012) 50-kHz calls in rats: effects of MDMA and the 5-HT(1A) receptor agonist 8-OH-DPAT. Pharmacol Biochem Behav 10:258–264
Savicky P (2014) pspearman: Spearman’s rank correlation test. R package version 0.3-0. https://CRAN.R-project.org/package=pspearman
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Scofield MD, Kalivas PW (2014) Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist 20(6):610–622
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Sengupta A, Bocchio M, Bannerman DM, Sharp T, Capogna M (2017) Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J Neurosci 37(7):1785–1796
Sesack SR, Grace AA (2010) Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35(1):27–47
Shalev U, Morales M, Hope B, Yap J, Shaham Y (2001) Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 156:98–107
Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55:1082–1089
Shinonaga Y, Takada M, Mizuno N (1994) Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience 58(2):389–397
Simola N, Fenu S, Costa G, Pinna A, Plumitallo A, Morelli M (2012) Pharmacological characterization of 50-kHz ultrasonic vocalizations in rats: comparison of the effects of different psychoactive drugs and relevance in drug-induced reward. Neuropharmacology 63:224–234
Sladeczek F, Pin JP, Recasens M, Bockaert J, Weiss S (1985) Glutamate stimulates inositol phosphate formation in striatal neurones. Nature 317:717–719
Stefaniuk M, Beroun A, Lebitko T, Markina O, Leski S, Meyza K, Grzywacz A, Samochowiec J, Samochowiec A, Radwanska K, Kaczmarek L (2017) Matrix metalloproteinase-9 and synaptic plasticity in the central amygdala in control of alcohol-seeking behavior. Biol Psychiatry 81(11):907–917
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Lehner M, Taracha E, Walkowiak J, Skórzewska A, Wisłowska-Stanek A, Hamed A, Bidziński A, Plaznik A (2008) Changes in the concentration of amino acids in the hippocampus of pentylenetetrazole-kindled rats. Neurosci Lett 439:245–249
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Bidziński A, Plaznik A (2010) Time course of changes in the concentrations of monoamines in the brain structures of pentylenetetrazole-kindled rats. J Neural Transm 117:707–718
Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ (2014) A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 19(4):888–900
Taracha E, Hamed A, Krząścik P, Lehner M, Skórzewska A, Płaźnik A, Chrapusta SJ (2012) Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague–Dawley rats. Psychopharmacology 222(4):619–632
Thompson B, Leonard KC, Brudzynski SM (2006) Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res 168(1):64–73
Tran L, Lasher BK, Young KA, Keele NB (2013) Depletion of serotonin in the basolateral amygdala elevates glutamate receptors and facilitates fear-potentiated startle. Transl Psychiatry 3:e298
Uejima JL, Bossert JM, Poles GC, Lu L (2007) Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res 181(2):292–296
Van Bockstaele EJ, Pickel VM (1993) Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol 334(4):603–617
Wang H, Liang S, Burgdorf J, Wess J, Yeomans J (2008) Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One 3(4):e1893
Wintink AJ, Brudzynski SM (2001) The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav 70(2–3):317–323
Wöhr M, Rippberger H, Schwarting RK, van Gaalen MM (2015) Critical involvement of 5-HT2C receptor function in amphetamine-induced 50-kHz ultrasonic vocalizations in rats. Psychopharmacology 232(10):1817–1829
Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365
Wolf ME, Tseng KY (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci 5:72
Wright MN, Ziegler A (2017) ranger: a fast implementation of random forests for high dimensional data in C++ and R. J Stat Softw 77(1):1–17
Wright JM, Gourdon JC, Clarke PB (2010) Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology 211(1):1–13
Wright JM, Dobosiewicz MR, Clarke PB (2012a) α- and β-adrenergic receptors differentially modulate the emission of spontaneous and amphetamine-induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology 37(3):808–821
Wright JM, Deng L, Clarke PB (2012b) Failure of rewarding and locomotor stimulant doses of morphine to promote adult rat 50-kHz ultrasonic vocalizations. Psychopharmacology (Berl) 224(4):477–487
Wright JM, Dobosiewicz MR, Clarke PB (2013) The role of dopaminergic transmission through D1-like and D2-like receptors in amphetamine-induced rat ultrasonic vocalizations. Psychopharmacology 225(4):853–868
Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA (2010) Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology 58(2):465–473
Zink M, Rapp S, Donev R, Gebicke-Haerter PJ, Thome J (2011) Fluoxetine treatment induces EAAT2 expression in rat brain. J Neural Transm 118(6):849–855
Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD (2011) Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. 14(5):620–626
Acknowledgements
This research was supported by Grant DEC-2011/03/B/NZ7/04155 from the National Science Centre, Poland. The study was supported by the Institute of Psychiatry and Neurology statutory found no. 501-003-13-043. We would like to thank Danuta Turzyńska and Alicja Sobolewska from Institute of Psychiatry and Neurology (Department of Neurochemistry) for HPLC analysis. Many thanks to Tomasz Jaroszewski for co-creation of RatRec software, who provided us with recording and detailed analysis of the USVs FFT spectrograms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest related to this work.
Ethical statement
The experiments were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609 EEC).
Study involving human and/or animal participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures using animal subjects were approved by the Local Committee for Animal Care and Use at Warsaw University of Medicine as compliant with Polish Law (21 of January 2005) (permission number: 20/2010). All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hamed, A., Kursa, M.B. Inter-individual differences in serotonin and glutamate co-transmission reflect differentiation in context-induced conditioned 50-kHz USVs response after morphine withdrawal. Brain Struct Funct 223, 3149–3167 (2018). https://doi.org/10.1007/s00429-018-1683-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1683-4