Abstract
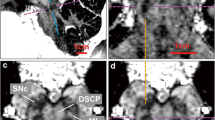
The pedunculopontine nucleus (PPN) has been proposed as target for deep brain stimulation (DBS) in patients with postural instability and gait disorders due to its involvement in muscle tonus adjustments and control of locomotion. However, it is a deep-seated brainstem nucleus without clear imaging or electrophysiological markers. Some studies suggested that diffusion tensor imaging (DTI) may help guiding electrode placement in the PPN by showing the surrounding fiber bundles, but none have provided a direct histological correlation. We investigated DTI fractional anisotropy (FA) maps from in vivo and in situ post-mortem magnetic resonance images (MRI) compared to histological evaluations for improving PPN targeting in humans. A post-mortem brain was scanned in a clinical 3T MR system in situ. Thereafter, the brain was processed with a special method ideally suited for cytoarchitectonic analyses. Also, nine volunteers had in vivo brain scanning using the same MRI protocol. Images from volunteers were compared to those obtained in the post-mortem study. FA values of the volunteers were obtained from PPN, inferior colliculus, cerebellar crossing fibers and medial lemniscus using histological data and atlas information. FA values in the PPN were significantly lower than in the surrounding white matter region and higher than in areas with predominantly gray matter. In Nissl-stained histologic sections, the PPN extended for more than 10 mm in the rostro-caudal axis being closely attached to the lateral parabrachial nucleus. Our DTI analyses and the spatial correlation with histological findings proposed a location for PPN that matched the position assigned to this nucleus in the literature. Coregistration of neuroimaging and cytoarchitectonic features can add value to help establishing functional architectonics of the PPN and facilitate neurosurgical targeting of this extended nucleus.










Similar content being viewed by others
References
Afshar F, Watkins ES, Yap JC (1978) Stereotaxic atlas of the human brainstem and cerebellar nuclei: a variability study. Raven Press, New York
Alegro M, Alho EJL, Lopes R, Zollei L, Amaro-Junior E (2015) A Computational Pipeline for Full Brain Histology to MRI registration. Organization for Human Brain Mapping, Honolulu, Hawaii, USA
Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative. Brain 12(1):26–41
Avants BB, Tustison N, Song G (2015) Advanced normalization tools (stnava.github.io/ants). USA
Benarroch EE (2013) Pedunculopontine nucleus: functional organization and clinical implications. Neurology 80(12):1148–1155
de Oliveira Souza C, de Lima-Pardini AC, Coelho DB, Brant R, Lopes-Alho EJ, Di Lorenzo-Alho AT, Teixeira LA, Teixeira MJ, Barbosa ER, Fonoff ET (2016) Pedunculopontine DBS improves balance in progressive supranuclear palsy: instrumental analysis. Clin Neurophys, doi:10.1016/j.clinph.2016.09.006 (In press)
Di Lorenzo Alho AT, Suemoto CK, Polichiso L, Tampellini E, de Oliveira KC, Molina M, Santos GA, Nascimento C, Leite RE, de Lucena Ferreti-Rebustini RE, da Silva AV, Nitrini R, Pasqualucci CA, Jacob-Filho W, Heinsen H, Grinberg LT (2015) Three-dimensional and stereological characterization of the human substantia nigra during aging. Brain Struct Funct doi:10.1007/s00429-015-1108-6
Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardès S, Pollak P (2010) Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 133:205–214
Ferretti REdL, Damin AE, Brucki SMD, Morillo LS, Perroco TR, Campora F, Moreira EG, Balbino ÉS, Lima MdCdA, Batella C, Ruiz L, Grinberg LT, Farfel JM, Leite REP, Suemoto CK, Pasqualucci CA, Rosemberg SR, Saldiva PHN, Jacob-Filho W, Nitrini R (2010) Post-mortem diagnosis of dementia by informant interview. Dement Neuropsychol 4(2):6
Fournier-Gosselin MP, Lipsman N, Saint-Cyr JA, Hamani C, Lozano AM (2013) Regional anatomy of the pedunculopontine nucleus: relevance for deep brain stimulation. Mov Disord 28(10):1330–1336
Fraix V, Bastin J, David O, Goetz L, Ferraye M, Benabid AL, Chabardes S, Pollak P, Debû B (2013) Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLoS One 8(12):e83919
Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, Nitrini R, Saldiva PH, Filho WJ, Group BABS (2007) Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank 8(2):151–162
Hamani C, Stone S, Laxton A, Lozano AM (2007) The pedunculopontine nucleus and movement disorders: anatomy and the role for deep brain stimulation. Parkinsonism Relat Disord 13(Suppl 3):S276–S280
Hamani C, Moro E, Lozano AM (2011) The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm 118(10):1461–1468
Hazrati LN, Parent A (1992) Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res 585(1–2):267–271
Heinsen H, Heinsen YL (1991) Serial thick, frozen, gallocyanin stained sections of human central nervous system. J Histotechnol 14(3):7
Heinsen H, Arzberger T, Schmitz C (2000) Celloidin mounting (embedding without infiltration)—a new, simple and reliable method for producing serial sections of high thickness through complete human brains and its application to stereological and immunohistochemical investigations. J Chem Neuroanat 20(1):49–59
Heinsen H, Arzberger T, Roggendorf W, Mitrovic T (2004) 3D reconstruction of celloidin-mounted serial sections. Acta Neuropathologica 108:374
Jenkinson N, Nandi D, Oram R, Stein JF, Aziz TZ (2006) Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. Neuroreport 17(6):639–641
Kang Y, Kitai ST (1990) Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res 535(1):79–95
Mazzone P, Insola A, Lozano A, Galati S, Scarnati E, Peppe A, Stanzione P, Stefani A (2007) Peripeduncular and pedunculopontine nuclei: a dispute on a clinically relevant target. Neuroreport 18(13):1407–1408
Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E (2008) Stereotactic surgery of nucleus tegmenti pedunculopontine [corrected]. Br J Neurosurg 22 Suppl 1:S33–40
Mazzone P, Garcia-Rill E, Scarnati E (2016) Progress in deep brain stimulation of the pedunculopontine nucleus and other structures: implications for motor and non-motor disorders. J Neural Transm (Vienna). doi:10.1007/s00702-016-1532-7
Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM (2010) Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain 133:215–224
Muthusamy KA, Aravamuthan BR, Kringelbach ML, Jenkinson N, Voets NL, Johansen-Berg H, Stein JF, Aziz TZ (2007) Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg 107(4):814–820
Olszewski J, Baxter D (1982) Cytoarchitecture of the Human Brain Stem. Karger, Basel, Switzerland
Plaha P, Gill SS (2005) Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport 16(17):1883–1887
Quester R, Schröder R (1997) The shrinkage of the human brain stem during formalin fixation and embedding in paraffin. J Neurosci Methods 75(1):81–89
Saper CB, Loewy AD (1982) Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res 252(2):367–372
Schulz G, Crooijmans HJ, Germann M, Scheffler K, Müller-Gerbl M, Müller B (2011) Three-dimensional strain fields in human brain resulting from formalin fixation. J Neurosci Methods 202(1):17–27
Simmons DM, Swanson LW (2009) Comparing histological data from different brains: sources of error and strategies for minimizing them. Brain Res Rev 60(2):349–367
Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P (2007) Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 2007130(6):1596–1607
Strauss I, Kalia SK, Lozano AM (2014) Where are we with surgical therapies for Parkinson’s disease? Parkinsonism Relat Disord 20(Suppl 1):S187–S191
Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, Silburn PA (2011a) Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69 (6):1248–1253 (discussion 1254)
Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, Silburn PA, Aziz TZ, Brown P (2011b) A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain 134:2085–2095
Yelnik J (2007) PPN or PPD, what is the target for deep brain stimulation in Parkinson’s disease? Brain 130:e79 (author reply e80)
Yeo SS, Kim SH, Ahn YH, Son SM, Jang SH (2011) Anatomical location of the pedunculopontine nucleus in the human brain: diffusion tensor imaging study. Stereotact Funct Neurosurg 89(3):152–156
Zrinzo L, Hariz M (2007) The peripeduncular nucleus: a novel target for deep brain stimulation? Neuroreport 18(15):1631–1632 (author reply 1632–1633)
Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, Hariz MI (2008) Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain 131(Pt 6):1588–1598
Zrinzo L, Zrinzo LV, Massey LA, Thornton J, Parkes HG, White M, Yousry TA, Strand C, Revesz T, Limousin P, Hariz MI, Holton JL (2011) Targeting of the pedunculopontine nucleus by an MRI-guided approach: a cadaver study. J Neural Transm 118(10):1487–1495
Acknowledgements
We are grateful to the volunteers who participated and family members who donated the brain for this study. We would also like to thank all the members of the Brain Bank of the Brazilian Aging Brain Study Group. Funding sources: Brazilian National Council for Scientific and Technological Development (CNPq), Institute for Education and Research of Albert Einstein Hospital, São Paulo Research Foundation (FAPESP), LIM-22 and LIM-44 (HC-FMUSP) for research financial and technical support in Brazil, and the National Institutes of Health, USA (R01AG040311).
Funding
Brazilian National Council for Scientific and Technological Development (CNPq), Institute for Education and Research of Albert Einstein Hospital, São Paulo Research Foundation (FAPESP), LIM-44 and LIM-22 (HC-FMUSP) for research financial and technical support in Brazil, and the National Institutes of Health, USA (R01AG040311).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Alho, A.T.D.L., Hamani, C., Alho, E.J.L. et al. Magnetic resonance diffusion tensor imaging for the pedunculopontine nucleus: proof of concept and histological correlation. Brain Struct Funct 222, 2547–2558 (2017). https://doi.org/10.1007/s00429-016-1356-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1356-0