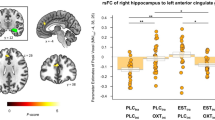
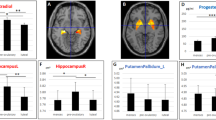
Abstract
There is substantial evidence that the ovarian sex hormones, estrogen and progesterone, which vary considerably over the course of the human female lifetime, contribute to changes in brain structure and function. This structured, quantitative literature reviews aims to summarize neuroimaging literature addressing physiological variation in brain macro- and microstructure across an array of hormonal transitions including the menstrual cycle, use of hormonal contraceptives, pregnancy, and menopause. Twenty-five studies reporting structural neuroimaging of women, addressing variation across hormonal states, were identified from a structured search of PUBMED and were systematically reviewed. Although the studies are heterogenous with regard to methodology, overall the results point to overlapping areas of hormone related effects on brain structure particularly affecting the structures of the limbic system. These findings are in keeping with functional data that point to a role for estrogen and progesterone in mediating emotional processing.


Similar content being viewed by others
Notes
Despite the relatively late maturation of the uncinate fasciculus, a review of DTI imaging examining the uncinate fasciculus across the lifespan did not identify sex differences in white matter microstructure (Hasan et al. 2009). This is at least suggestive that there may not be a robust relationship between myelination of this structure and the sex hormones.
For further review of functional neuroimaging studies investigating the menstrual cycle and OCP use see Sacher et al. (2013) and Toffoletto et al. (2014). For further review of the putative roles of neurosteroids in symptomatology of PMS, pregnancy, the postpartum period, and menopause, see Pluchino et al. (2013).
Cohen’s d is commonly used to classify the effect size as small (<00.4), medium (0.4–0.7), or large (>0.7) (Rose and Donohoe 2013).
Abbreviations
- ACC:
-
Anterior cingulate cortex
- ADC:
-
Apparent diffusion coefficient
- ALLO:
-
Allopregnanolone
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- EE:
-
Ethinyl estradiol
- ER:
-
Estrogen receptor
- FDR:
-
False discovery rate
- fMRI:
-
Functional magnetic resonance imaging
- FSH:
-
Follicle stimulating hormone
- GABA:
-
Gamma-aminobutryic acid
- GM:
-
Grey matter
- GnRH:
-
Gonadotropin releasing hormone
- GPCR:
-
G-protein coupled receptor
- HRT:
-
Hormone replacement therapy
- IUD:
-
Intrauterine device
- LH:
-
Luteinizing hormone
- MD:
-
Mean diffusivity
- MNI:
-
Montreal Neurological Institute
- MRI:
-
Magnetic resonance imaging
- mRNA:
-
Messenger ribonucleic acid
- OCP:
-
Oral contraceptive pills
- OFC:
-
Orbitofrontal cortex
- PD:
-
Primary dysmenorrhea
- PFC:
-
Prefrontal cortex
- PMDD:
-
Premenstrual dysphoric disorder
- PMS:
-
Premenstrual syndrome
- ROI:
-
Region of interest
- VBM:
-
Voxel based morphometry
- WM:
-
White matter
References
Abler B, Kumpfmuller D, Gron G, Walter M, Stingl J, Seeringer A (2013) Neural correlates of erotic stimulation under different levels of female sexual hormones. PloS one 8:e54447. doi:10.1371/journal.pone.0054447
Abraham GE, Odell WD, Swerdloff RS, Hopper K (1972) Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17 beta during the menstrual cycle. J Clin Endocrinol Metabol 34:312–318. doi:10.1210/jcem-34-2-312
Amin Z, Epperson CN, Constable RT, Canli T (2006) Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage 32:457–464. doi:10.1016/j.neuroimage.2006.03.013
Aminoff EM, Kveraga K, Bar M (2013) The role of the parahippocampal cortex in cognition. Trends in cognitive sciences 17:379–390. doi:10.1016/j.tics.2013.06.009
Andreano JM, Cahill L (2009) Sex influences on the neurobiology of learning and memory. Learn Memory 16:248–266. doi:10.1101/lm.918309
Arnold AP, Gorski RA (1984) Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7:413–442. doi:10.1146/annurev.ne.07.030184.002213
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. NeuroImage 11:805–821. doi:10.1006/nimg.2000.0582
Backstrom T et al (2003) Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci 1007:42–53
Banks WA (2012) Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology 153:4111–4119. doi:10.1210/en.2012-1435
Baroncini M, Jissendi P, Catteau-Jonard S, Dewailly D, Pruvo JP, Francke JP, Prevot V (2010) Sex steroid hormones-related structural plasticity in the human hypothalamus. NeuroImage 50:428–433. doi:10.1016/j.neuroimage.2009.11.074
Bergouignan L et al (2009) Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? NeuroImage 45:29–37. doi:10.1016/j.neuroimage.2008.11.006
Biggs WS, Demuth RH (2011) Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician 84:918–924
Blaustein JD (2008) An estrogen by any other name. Endocrinology 149:2697–2698. doi:10.1210/en.2008-0396
Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y (2011) Diffusion MRI of structural brain plasticity induced by a learning and memory task. PloS One 6:e20678. doi:10.1371/journal.pone.0020678
Braun K (2011) The prefrontal-limbic system: development, neuroanatomy, function, and implications for socioemotional development. Clin Perinatol 38:685–702. doi:10.1016/j.clp.2011.08.013
Breedlove SM, Arnold AP (1980) Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science 210:564–566
Brinton RD et al (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339. doi:10.1016/j.yfrne.2008.02.001
Burgess N, Maguire EA, O’Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641
Catani M, Dell’acqua F, Thiebaut de Schotten M (2013) A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37:1724–1737. doi:10.1016/j.neubiorev.2013.07.001
Chen W, Shields J, Huang W, King JA (2009) Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav Brain Res 201:8–13. doi:10.1016/j.bbr.2009.01.019
Christensen A, Dewing P, Micevych P (2011) Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci Off J Soc Neurosci 31:17583–17589. doi:10.1523/JNEUROSCI.3030-11.2011
Cooke BM (2006) Steroid-dependent plasticity in the medial amygdala. Neuroscience 138:997–1005. doi:10.1016/j.neuroscience.2005.06.018
Cooke BM, Woolley CS (2005) Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46. doi:10.1002/neu.20143
Cooke BM, Tabibnia G, Breedlove SM (1999) A brain sexual dimorphism controlled by adult circulating androgens. Proc Nat Acad Sci USA 96:7538–7540
Cooke BM, Breedlove SM, Jordan CL (2003) Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav 43:336–346
Dastidar P et al (1999) Volumes of brain atrophy and plaques correlated with neurological disability in secondary progressive multiple sclerosis. J Neurol Sci 165:36–42
Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR (2009) Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology 51:491–503. doi:10.1007/s00234-009-0521-z
De Bondt T, Jacquemyn Y, Van Hecke W, Sijbers J, Sunaert S, Parizel PM (2013a) Regional gray matter volume differences and sex-hormone correlations as a function of menstrual cycle phase and hormonal contraceptives use. Brain Res 1530:22–31. doi:10.1016/j.brainres.2013.07.034
De Bondt T et al (2013b) Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Prelim Results DTI Tract Study Eur Radiol 23:57–64. doi:10.1007/s00330-012-2572-5
den Heijer T, Geerlings MI, Hofman A, de Jong FH, Launer LJ, Pols HA, Breteler MM (2003) Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol 60:213–220
Dinc H, Esen F, Demirci A, Sari A, Resit Gumele H (1998) Pituitary dimensions and volume measurements in pregnancy and post partum. MR Assess Acta (Radiol Stockholm Sweden, 1987) 39:64–69
Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004) Neuroplasticity: changes in grey matter induced by training. Nature 427:311–312. doi:10.1038/427311a
Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF (2007) Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 104:2465–2470. doi:10.1073/pnas.0605569104
Elster AD, Sanders TG, Vines FS, Chen MY (1991) Size and shape of the pituitary gland during pregnancy and post partum: measurement with MR imaging. Radiology 181:531–535. doi:10.1148/radiology.181.2.1924800
Epstein R, Kanwisher N (1998) A cortical representation of the local visual environment. Nature 392:598–601. doi:10.1038/33402
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. doi:10.1038/3305
Fan L, Hanbury R, Pandey SC, Cohen RS (2008) Dose and time effects of estrogen on expression of neuron-specific protein and cyclic AMP response element-binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology 88:111–126. doi:10.1159/000129498
Faundes A, Segal SJ, Adejuwon CA, Brache V, Leon P, Alvarez-Sanchez F (1980) The menstrual cycle in women using an intrauterine device. Fertil Steril 34:427–430
Featherstone RE, Fleming AS, Ivy GO (2000) Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behav Neurosci 114:158–172
Filipek PA, Richelme C, Kennedy DN, Caviness VS (1994) The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex (New York NY 1991) 4:344–360
Fischer B, Gleason C, Asthana S (2014) Effects of hormone therapy on cognition and mood. Fertil Steril 101:898–904. doi:10.1016/j.fertnstert.2014.02.025
Fowler CD, Freeman ME, Wang Z (2003) Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol 57:257–269. doi:10.1002/neu.10273
Franke K, Hagemann G, Schleussner E, Gaser C (2015) Changes of individual BrainAGE during the course of the menstrual cycle. NeuroImage. doi:10.1016/j.neuroimage.2015.04.036
Frisk V, Milner B (1990) The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia 28:349–359
Gillies GE, McArthur S (2010) Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62:155–198. doi:10.1124/pr.109.002071
Gillings MR (2014) Were there evolutionary advantages to premenstrual syndrome? Evol Appl 7:897–904. doi:10.1111/eva.12190
Goldstein JM et al (2001) Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex (New York NY 1991) 11:490–497
Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N (2005) Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci Off J Soc Neurosci 25:9309–9316. doi:10.1523/JNEUROSCI.2239-05.2005
Gonzalez JG, Elizondo G, Saldivar D, Nanez H, Todd LE, Villarreal JZ (1988) Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med 85:217–220
Goto M et al (2011a) 3 Tesla MRI detects accelerated hippocampal volume reduction in postmenopausal women. J Magn Reson Imaging JMRI 33:48–53. doi:10.1002/jmri.22328
Goto M et al (2011b) Accelerated hippocampal volume reduction in post-menopausal women: an additional study with Atlas-based method. Radiol Phys Technol 4:185–188. doi:10.1007/s12194-011-0120-7
Grams AE, Gempt J, Stahl A, Forschler A (2010) Female pituitary size in relation to age and hormonal factors. Neuroendocrinology 92:128–132. doi:10.1159/000314196
Grant R, Condon B, Lawrence A, Hadley DM, Patterson J, Bone I, Teasdale GM (1988) Is cranial CSF volume under hormonal influence? An MR study. J Comput Assist Tomogr 12(1):36–39
Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I (2002) Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull 59:105–109
Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, Gaser C (2011) Changes in brain size during the menstrual cycle. PloS One 6:e14655. doi:10.1371/journal.pone.0014655
Ham EA, Cirillo VJ, Zanetti ME, Kuehl FA Jr (1975) Estrogen-directed synthesis of specific prostaglandins in uterus. Proc Natl Acad Sci USA 72:1420–1424
Harris GW (1970) Effects of the nervous system on the pituitary-adrenal activity. Prog Brain Res 32:86–88
Harris RJ, Rice GE, Young AW, Andrews TJ (2015) Distinct but overlapping patterns of response to words and faces in the fusiform gyrus. Cereb Cortex (New York, NY 1991). doi:10.1093/cercor/bhv147
Hasan KM et al (2009) Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res 1276:67–76. doi:10.1016/j.brainres.2009.04.025
Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH (2001) Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metabol 86:4798–4804. doi:10.1210/jcem.86.10.7919
Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005) Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–1213. doi:10.1016/j.pnpbp.2005.08.006
Herson PS, Koerner IP, Hurn PD (2009) Sex, sex steroids, and brain injury. Sem Reprod Med 27:229–239
Hinshaw DB Jr, Hasso AN, Thompson JR, Davidson BJ (1984) High resolution computed tomography of the post partum pituitary gland. Neuroradiology 26:299–301
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. doi:10.1038/nature14188
Jeong HG, Ham BJ, Yeo HB, Jung IK, Joe SH (2012) Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord 140:260–267. doi:10.1016/j.jad.2012.02.010
Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci Off J Soc Neurosci 17:4302–4311
Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F (2010) Pregnancy and pituitary disorders. Eur J Endocrinol Eur Fed Endocr Soc 162:453–475. doi:10.1530/EJE-09-0923
Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE (2010) The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci 124:695–700. doi:10.1037/a0020884
Kondo Y, Sachs BD, Sakuma Y (1997) Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res 88:153–160
Konrad C et al (2008) The functional anatomy of semantic retrieval is influenced by gender, menstrual cycle, and sex hormones. J Neural Transm 115:1327–1337. doi:10.1007/s00702-008-0073-0
Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF (2002) Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol 454:115–139. doi:10.1002/cne.10416
Lai CH (2013) Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res 211:37–46. doi:10.1016/j.pscychresns.2012.06.006
Laule C, Leung E, Lis DK, Traboulsee AL, Paty DW, MacKay AL, Moore GR (2006) Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler 12:747–753
Lieberburg I, McEwen BS (1975) Estradiol-17beta: a metabolite of testosterone recovered in cell nuclei from limbic areas of adult male rat brains. Brain Res 91:171–174
Lisofsky N, Lindenberger U, Kuhn S (2015a) Amygdala/hippocampal activation during the menstrual cycle: evidence for lateralization of effects across different tasks. Neuropsychologia 67:55–62. doi:10.1016/j.neuropsychologia.2014.12.005
Lisofsky N, Martensson J, Eckert A, Lindenberger U, Gallinat J, Kuhn S (2015b) Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage 118:154–162. doi:10.1016/j.neuroimage.2015.06.012
Madeira MD, Ferreira-Silva L, Paula-Barbosa MM (2001) Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol 432:329–345
McEwen BS, Pfaff DW, Chaptal C, Luine VN (1975) Brain cell nuclear retention of [3H]estradiol doses able to promote lordosis: temporal and regional aspects. Brain Res 86:155–161
McEwen BS, Gray JD, Nasca C (2015) 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol 226:T67–T83. doi:10.1530/JOE-15-0121
Meyer G, Wahle P, Castaneyra-Perdomo A, Ferres-Torres R (1992) Morphology of neurons in the white matter of the adult human neocortex. Exp Brain Res 88:204–212
Micevych P, Christensen A (2012) Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol 33:331–341. doi:10.1016/j.yfrne.2012.07.003
Miki Y et al (2005) The pituitary gland: changes on MR images during the 1st year after delivery. Radiology 235:999–1004. doi:10.1148/radiol.2353040243
Montagnese CM, Poulain DA, Vincent JD, Theodosis DT (1987) Structural plasticity in the rat supraoptic nucleus during gestation, post-partum lactation and suckling-induced pseudogestation and lactation. J Endocrinol 115:97–105
Naftolin F et al (2007) Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci 14:101–116. doi:10.1177/1933719107301059
Nakata H, Sakamoto K, Kakigi R (2014) Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol 5:1489. doi:10.3389/fpsyg.2014.01489
Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, Bydder GM (2002) Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. AJNR Am J Neuroradiol 23:19–26
Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G (2010) Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology 35:47–55. doi:10.1016/j.psyneuen.2009.08.011
Ossewaarde L, van Wingen GA, Rijpkema M, Backstrom T, Hermans EJ, Fernandez G (2013) Menstrual cycle-related changes in amygdala morphology are associated with changes in stress sensitivity. Hum Brain Mapp 34:1187–1193. doi:10.1002/hbm.21502
Palumbo MA et al (1995) Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Invest 18:853–856
Paton JJ, Belova MA, Morrison SE, Salzman CD (2006) The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439:865–870. doi:10.1038/nature04490
Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J (2011) Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology 36:1101–1113. doi:10.1016/j.psyneuen.2011.05.004
Peterson BM, Mermelstein PG, Meisel RL (2015) Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220:2415–2422. doi:10.1007/s00429-014-0794-9
Phoenix CH, Goy RW, Gerall AA, Young WC (1959) Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382. doi:10.1210/endo-65-3-369
Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH (2010) Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res 1348:55–62. doi:10.1016/j.brainres.2010.06.019
Pletzer B, Kronbichler M, Kerschbaum H (2015) Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volume and face recognition performance. Brain Res 1596:108–115. doi:10.1016/j.brainres.2014.11.025
Pluchino N et al (2006) Progesterone and progestins: effects on brain, allopregnanolone and beta-endorphin. J Steroid Biochem Mol Biol 102:205–213. doi:10.1016/j.jsbmb.2006.09.023
Pluchino N, Santoro A, Casarosa E, Wenger JM, Genazzani AD, Petignat P, Genazzani AR (2013) Advances in neurosteroids: role in clinical practice. Climact J Int Menopause Soc 16(Suppl 1):8–17
Poromaa IS (2014) Physiological correlates of premenstrual dysphoric disorder (PMDD). Curr Topics Behav Neurosci. doi:10.1007/7854_2014_296
Protopopescu X et al (2005) Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA 102:16060–16065. doi:10.1073/pnas.0502818102
Protopopescu X et al (2008a) Hippocampal structural changes across the menstrual cycle. Hippocampus 18:985–988. doi:10.1002/hipo.20468
Protopopescu X et al (2008b) Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 108:87–94. doi:10.1016/j.jad.2007.09.015
Qiu LR, Germann J, Spring S, Alm C, Vousden DA, Palmert MR, Lerch JP (2013) Hippocampal volumes differ across the mouse estrous cycle, can change within 24 hours, and associate with cognitive strategies. NeuroImage 83:593–598. doi:10.1016/j.neuroimage.2013.06.074
Ridgway GR, Henley SM, Rohrer JD, Scahill RI, Warren JD, Fox NC (2008) Ten simple rules for reporting voxel-based morphometry studies. NeuroImage 40:1429–1435. doi:10.1016/j.neuroimage.2008.01.003
Rolls ET (2000) The orbitofrontal cortex and reward. Cereb cortex (New York, NY: 1991) 10:284–294
Rose EJ, Donohoe G (2013) Brain vs. behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull 39:518–526. doi:10.1093/schbul/sbs056
Rosenberg L, Park S (2002) Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology 27:835–841
Rosenthal R, DiMatteo MR (2001) Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol 52:59–82. doi:10.1146/annurev.psych.52.1.59
Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J (2014) A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39:34–50. doi:10.1016/j.neubiorev.2013.12.004
Sacher J, Okon-Singer H, Villringer A (2013) Evidence from neuroimaging for the role of the menstrual cycle in the interplay of emotion and cognition. Front Human Neurosci 7:374. doi:10.3389/fnhum.2013.00374
Sanchez AM, Flamini MI, Genazzani AR, Simoncini T (2013) Effects of progesterone and medroxyprogesterone on actin remodeling and neuronal spine formation. Mol Endocrinol 27:693–702. doi:10.1210/me.2012-1278
Scholz J, Klein MC, Behrens TE, Johansen-Berg H (2009) Training induces changes in white-matter architecture. Nat Neurosci 12:1370–1371. doi:10.1038/nn.2412
Schoning S et al (2007) Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia 45:3203–3214. doi:10.1016/j.neuropsychologia.2007.06.011
Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK (2006) Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci Off J Soc Neurosci 26:10789–10795. doi:10.1523/JNEUROSCI.2577-06.2006
Siegel HI, Rosenblatt JS (1975) Estrogen-induced maternal behavior in hysterectomized-overiectomized virgin rats. Physiol Behav 14:465–471
Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95. doi:10.1002/cne.902940107
Skalkidou A, Hellgren C, Comasco E, Sylven S, Sundstrom Poromaa I (2012) Biological aspects of postpartum depression. Women’s Health 8:659–672. doi:10.2217/whe.12.55
Smith ML, Milner B (1981) The role of the right hippocampus in the recall of spatial location. Neuropsychologia 19:781–793
Soares CN, Frey BN (2010) Challenges and opportunities to manage depression during the menopausal transition and beyond. Psychiatr Clin N Am 33:295–308. doi:10.1016/j.psc.2010.01.007
Srivastava DP, Woolfrey KM, Penzes P (2013) Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev 65:1318–1350. doi:10.1124/pr.111.005272
Stanczyk FZ, Archer DF, Bhavnani BR (2013) Ethinyl estradiol and 17beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 87:706–727. doi:10.1016/j.contraception.2012.12.011
Sullivan EV, Marsh L, Pfefferbaum A (2005) Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging 26:1093–1098. doi:10.1016/j.neurobiolaging.2004.09.015
Sundstrom Poromaa I, Gingnell M (2014) Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci 8:380. doi:10.3389/fnins.2014.00380
Suzuki R, Rygh LJ, Dickenson AH (2004) Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci 25:613–617. doi:10.1016/j.tips.2004.10.002
Teasdale GM, Grant R, Condon B, Patterson J, Lawrence A, Hadley DM, Wyper D (1988) Intracranial CSF volumes: natural variations and physiological changes measured by MRI. Acta neurochir Suppl (Wien) 42:230–235
Toffoletto S, Lanzenberger R, Gingnell M, Sundstrom-Poromaa I, Comasco E (2014) Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology 50:28–52. doi:10.1016/j.psyneuen.2014.07.025
Torrealday S, Taylor HS, Burney RO, Mooney SB, Giudice LC (2000) Endocrinology of Pregnancy. In: De Groot LJ et al. (eds) Endotext. South Dartmouth, MA
Tu CH et al (2010) Brain morphological changes associated with cyclic menstrual pain. Pain 150:462–468. doi:10.1016/j.pain.2010.05.026
Tu CH et al (2013) Menstrual pain is associated with rapid structural alterations in the brain. Pain 154:1718–1724. doi:10.1016/j.pain.2013.05.022
van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC (2005) Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 72:168–174. doi:10.1016/j.contraception.2005.03.005
Warren AM, Gurvich C, Worsley R, Kulkarni J (2014) A systematic review of the impact of oral contraceptives on cognition. Contraception 90:111–116. doi:10.1016/j.contraception.2014.03.015
Whitwell JL (2009) Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J Neurosci Off J Soc Neurosci 29:9661–9664. doi:10.1523/JNEUROSCI.2160-09.2009
Wideman L, Montgomery MM, Levine BJ, Beynnon BD, Shultz SJ (2013) Accuracy of calendar-based methods for assigning menstrual cycle phase in women. Sports Health 5:143–149. doi:10.1177/1941738112469930
Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R (2010) Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. NeuroImage 49:1205–1212. doi:10.1016/j.neuroimage.2009.09.046
Wong AP et al (2014) Estimating volumes of the pituitary gland from T1-weighted magnetic-resonance images: effects of age, puberty, testosterone, and estradiol. NeuroImage 94:216–221. doi:10.1016/j.neuroimage.2014.02.030
Woolley CS, McEwen BS (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci Off J Soc Neurosci 12:2549–2554
Xiao B, Zeng T, Wu S, Sun H, Xiao N (1995) Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception 51:359–365
Ylikorkala O, Puolakka J, Kauppila A (1979) Serum gonadotrophins, prolactin and ovarian steroids in primary dysmenorrhoea. Br J Obstet Gynaecol 86:648–653
Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. doi:10.1038/nn.3045
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catenaccio, E., Mu, W. & Lipton, M.L. Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct 221, 3845–3867 (2016). https://doi.org/10.1007/s00429-016-1197-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1197-x