Abstract
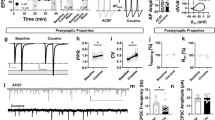
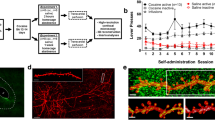
Animal models of relapse reveal that the motivation to seek drug is regulated by enduring morphological and physiological changes in the nucleus accumbens, as well as transient synaptic potentiation in the accumbens core (NAcore) that parallels drug-seeking behavior. The current study sought to examine the link between the behavioral and synaptic consequences of cue-induced cocaine seeking by optically silencing glutamatergic afferents to the NAcore from the prelimbic cortex (PL). Adeno-associated virus coding for the inhibitory opsin archaerhodopsin was microinjected into PL, and optical fibers were targeted to NAcore. Animals were trained to self-administer cocaine followed by extinction training, and then underwent cue-induced reinstatement in the presence or absence of 15 min of optically induced inhibition of PL fibers in NAcore. Inhibiting the PL-to-NAcore projection blocked reinstated behavior and was paralleled by decreased dendritic spine head diameter and AMPA/NMDA ratio relative to sham-laser control rats. Interestingly, while spine density was elevated after extinction training, no further effects were observed by cued reinstatement or optical inhibition. These findings validate the critical role for PL afferents to the NAcore in simultaneously regulating both reinstated behavior and the associated transient synaptic potentiation.



Similar content being viewed by others
References
Adamantidis AR et al (2011) Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci 31:10829–10835. doi:10.1523/JNEUROSCI.2246-11.2011
Alhaddad H, Das SC, Sari Y (2014) Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. doi:10.1007/s00213-014-3545-y
Bhatt DH, Zhang S, Gan WB (2009) Dendritic spine dynamics. Annu Rev Physiol 71:261–282. doi:10.1146/annurev.physiol.010908.163140
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76:790–803. doi:10.1016/j.neuron.2012.09.040
Chow BY et al (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463:98–102. doi:10.1038/nature08652
Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24:7167–7173. doi:10.1523/JNEUROSCI.1581-04.2004
Dietz DM et al (2012) Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci 15:891–896. doi:10.1038/nn.3094
Dumitriu D et al (2012) Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci 32:6957–6966. doi:10.1523/JNEUROSCI.5718-11.2012
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759
Fuchs RA, Eaddy JL, Su ZI, Bell GH (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26:487–498
Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW (2013a) Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron 77:867–872. doi:10.1016/j.neuron.2013.01.005
Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013b) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA 110:9124–9129. doi:10.1073/pnas.1220591110
Golden SA, Russo SJ (2012) Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harbor Perspect Med. doi:10.1101/cshperspect.a011957
Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT (2013) Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc Natl Acad Sci USA 110:3597–3602. doi:10.1073/pnas.1219593110
Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650. doi:10.1016/j.neuron.2005.02.005
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. doi:10.1038/npp.2009.110
LaLumiere RT, Smith KC, Kalivas PW (2012) Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci 35:614–622. doi:10.1111/j.1460-9568.2012.07991.x
Li J, Liu N, Lu K, Zhang L, Gu J, Guo F, An S (2012) Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neurosci Lett 517:118–122. doi:10.1016/j.neulet.2012.04.040
Luscher C, Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69:650–663. doi:10.1016/j.neuron.2011.01.017
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537
McGinty JF, Whitfield TW Jr, Berglind WJ (2010) Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314:183–193. doi:10.1016/j.brainres.2009.08.078
Olive MF (2009) Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev 2:83–98
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier Academic Press, Burlington
Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW (2014) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. doi:10.1111/adb.12127
Robinson TE, Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Suppl 1):33–46
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ (2010) The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33:267–276. doi:10.1016/j.tins.2010.02.002
Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL (2011) Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol 46:239–246. doi:10.1093/alcalc/agr023
Shen H, Sesack SR, Toda S, Kalivas PW (2008) Automated quantification of dendritic spine density and spine head diameter in medium spiny neurons of the nucleus accumbens. Brain Struct Funct 213:149–157. doi:10.1007/s00429-008-0184-2
Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW (2009) Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci 29:2876–2884. doi:10.1523/JNEUROSCI.5638-08.2009
Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW (2011) Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA 108:19407–19412. doi:10.1073/pnas.1112052108
Shen H-W, Gipson CD, Huits M, Kalivas PW (2014a) Prelimbic cortex and ventral tegmental area modulate synaptic plasticity in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology (in press)
Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW (2014b) Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci 34:5649–5657. doi:10.1523/JNEUROSCI.4564-13.2014
Smith RJ, Lobo MK, Spencer S, Kalivas PW (2013) Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol 23:546–552. doi:10.1016/j.conb.2013.01.026
Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD (2012) Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc 7:12–23. doi:10.1038/nprot.2011.413
Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD (2013) Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addict Biol. doi:10.1111/adb.12064
Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD (2014) Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addict Biol. doi:10.1111/adb.12064
Stefanik MT, Kalivas PW (2013) Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci 7:213. doi:10.3389/fnbeh.2013.00213
Stefanik MT, Kupchik YM, Brown RM, Kalivas PW (2013a) Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci 33:13654–13662. doi:10.1523/JNEUROSCI.1570-13.2013
Stefanik MT et al (2013b) Optogenetic inhibition of cocaine seeking in rats. Addict Biol 18:50–53. doi:10.1111/j.1369-1600.2012.00479.x
Stuber GD et al (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. doi:10.1038/nature10194
Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084. doi:10.1126/science.1168878
Tsunematsu T, Tabuchi S, Tanaka KF, Boyden ES, Tominaga M, Yamanaka A (2013) Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res. doi:10.1016/j.bbr.2013.05.021
Wolf ME (2010) The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33:391–398. doi:10.1016/j.tins.2010.06.003
Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011) Optogenetics in neural systems. Neuron 71:9–34. doi:10.1016/j.neuron.2011.06.004
Acknowledgments
This work was supported by National Institutes of Health T32 DA7288 and Grants DA015369, DA012513, and DA003906 (P.W.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefanik, M.T., Kupchik, Y.M. & Kalivas, P.W. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct 221, 1681–1689 (2016). https://doi.org/10.1007/s00429-015-0997-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-0997-8