Abstract
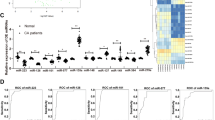
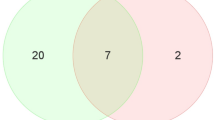
Arterial calcification is an actively regulated process, with different morphological manifestations. Micro-RNAs emerged as potential regulators of vascular calcification; they may become novel diagnostic tools and be used for a finest staging of the carotid plaque progression. The present study aimed at characterizing the different miRNA-mRNA axes in carotid plaques according to their histological patterns of calcification. Histopathological analysis was performed on 124 retrospective carotid plaques, with clinical data and preoperatory angio-CT. miRNA analysis was carried out with microfluidic cards. Real-time PCR was performed for selected miRNAs validation and for RUNX-2 and SOX-9 mRNA levels. CD31, CD68, SMA, and SOX-9 were analyzed by immunohistochemistry. miRNA levels on HUVEC cells were analyzed for confirming results under in vitro osteogenic conditions. Histopathological analysis revealed two main calcification subtypes of plaques: calcific cores (CC) and protruding nodules (PN). miRNA array and PCR validation of miR-1275, miR-30a-5p, and miR-30d indicated a significant upregulation of miR-30a-5p and miR-30d in the PN plaques. Likewise, the miRNA targets RUNX-2 and SOX-9 resulted poorly expressed in PN plaques. The inverse correlation between miRNA and RUNX-2 levels was confirmed on osteogenic-differentiated HUVEC. miR-30a-5p and miR-30d directly correlated with calcification extension and thickness at angio-CT imaging. Our study demonstrated the presence of two distinct morphological subtypes of calcification in carotid atheromatous plaques, supported by different miRNA signatures, and by different angio-CT features. These results shed the light on the use of miRNA as novel diagnostic markers, suggestive of plaque evolution.




Similar content being viewed by others
References
Hofmann Bowman MA, McNally EM (2012) Genetic pathways of vascular calcification. Trends Cardiovasc Med 22:93–98. https://doi.org/10.1016/j.tcm.2012.07.002
Villa-Bellosta R, Egido J (2017) Phosphate, pyrophosphate, and vascular calcification: a question of balance. Eur Heart J 38:1801–1804. https://doi.org/10.1093/eurheartj/ehv605
Vasuri F, Fittipaldi S, Pasquinelli G (2014) Arterial calcification: finger-pointing at resident and circulating stem cells. World J Stem Cells 6:540–551. https://doi.org/10.4252/wjsc.v6.i5.540
Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Boström K, Demer LL (2003) Multilineage potential of cells from the artery wall. Circulation 108:2505–2510. https://doi.org/10.1161/01.CIR.0000096485.64373.C5
Zhu D, Mackenzie NC, Farquharson C, MacRae VE (2012) Mechanisms and clinical consequences of vascular calcification. Front Endocrinol (Lausanne) 3:95. https://doi.org/10.3389/fendo.2012.00095
Maldonado N, Kelly-Arnold A, Vengrenyuk Y, Laudier D, Fallon JT, Virmani R, Cardoso L, Wienbaum S (2012) A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: potential implications for plaque rupture. Am J Physiol Heart Circ Physiol 303:H619–H628. https://doi.org/10.1152/ajpheart.00036.2012
Imoto K, Hiro T, Fujii T, Murashige A, Fukumoto Y, Hashimoto G, Okamura T, Yamada J, Mori K, Matsuzaki M (2005) Longitudinal structural determinants of atherosclerotic plaque vulnerability: a computational analysis of stress distribution using vessel models and three-dimensional intravascular ultrasound imaging. J Am Coll Cardiol 46:1507–1515. https://doi.org/10.1016/j.jacc.2005.06.069
Wong KK, Thavornpattanapong P, Cheung SC, Sun Z, Tu J (2012) Effect of calcification on the mechanical stability of plaque based on a three-dimensional carotid bifurcation model. BMC Cardiovasc Disord 12:7–18. https://doi.org/10.1186/1471-2261-12-7
Holzapfel GA, Mulvihill JJ, Cunnane EM, Walsh MT (2014) Computational approaches for analyzing the mechanics of atherosclerotic plaques: a review. J Biomech 47:859–869. https://doi.org/10.1016/j.jbiomech.2014.01.011
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association. Circulation 92:1355–1374
Stary HC (2000) Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 20:1177–1178
Vasuri F, Fittipaldi S, Pini R, Degiovanni A, Mauro R, D'Errico-Grigioni A, Faggioli G, Stella A, Pasquinelli G (2015) Diffuse calcifications protect carotid plaques regardless of the amount of neoangiogenesis and related histological complications. Biomed Res Int 2015:795672–795678. https://doi.org/10.1155/2015/795672
Pini R, Faggioli G, Fittipaldi S, Vasuri F, Longhi M, Gallitto E, Pasquinelli G, Gargiulo M, Stella A (2017) Relationship between calcification and vulnerability of the carotid plaques. Ann Vasc Surg 44:336–342. https://doi.org/10.1016/j.avsg.2017.04.017
Zhou S, Jin J, Wang J, Zhang Z, Freedman JH, Zheng Y, Cai L (2018) miRNAs in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin 39:1073–1084. https://doi.org/10.1038/aps.2018.30
Balderman JAF, Lee HY, Mahoney CE, Handy DE, White K, Annis S, Lebeche D, Hajjar RJ, Loscalzo J, Leopold JA (2012) Bone morphogenetic protein-2 decreases microrna -30b and microrna -30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc 1:e003905. https://doi.org/10.1161/JAHA.112.003905
Goettsch C, Hutcheson JD, Aikawa E (2013) MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circ Res 112:1073–1084. https://doi.org/10.1161/CIRCRESAHA.113.300937
Bidzhekov K, Gan L, Denecke B, Rostalsky A, Hristov M, Koeppel TA, Zernecke A, Weber C (2012) Microrna expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thromb Haemost 107:619–625. https://doi.org/10.1160/TH11-09-0607
Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R (2014) Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol 34:724–736
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Ciavarella C, Gallitto E, Ricci F, Buzzi M, Stella A, Pasquinelli G (2017) The crosstalk between vascular MSCs and inflammatory mediators determines the pro-calcific remodelling of human atherosclerotic aneurysm. Stem Cell Res Ther 8:99. https://doi.org/10.1186/s13287-017-0554-x
Babiarz LS, Yousem DM, Bilker W, Wasserman BA (2005) Middle cerebral artery infarction: relationship of cavenous carotid artery calcification. AJNR Am J Neuroradiol 26:1505e11
Shesh NR, Ray HE, Yuan X, Pan J, Hamid T, Prabhu SD (2012) Statistical analysis of repeated microRNA high-throughput data with application to human heart failure: a review of methodology. Open Access Med Stat 2012:21–31. https://doi.org/10.2147/OAMS.S27907
Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK (2013) OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One 8:e58796. https://doi.org/10.1371/journal.pone.0058796
Naqvi AR, Fordham JB, Nares S (2015) miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J Immunol 194:1916–1927. https://doi.org/10.4049/jimmunol.1401893
Collett GD, Canfield AE (2005) Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res 96:930–938. https://doi.org/10.1161/01.RES.0000163634.51301.0d
New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E (2013) Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res 113:72–77. https://doi.org/10.1161/CIRCRESAHA.113.301036
Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, Harmsen MC (2015) Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 108:377–386. https://doi.org/10.1093/cvr/cvv175
Pang L, You L, Ji C, Shi C, Chen L, Yang L, Huang F, Zhou Y, Zhang J, Chen X, Guo X (2016) miR-1275 inhibits adipogenesis via ELK1 and its expression decreases in obese subjects. J Mol Endocrinol 57:33–43. https://doi.org/10.1530/JME-16-0007
Maitrias P, Metzinger-Le Meuth V, Massy ZA, M'Baya-Moutoula E, Reix T, Caus T, Metzinger L (2015) MicroRNA deregulation in symptomatic carotid plaque. J Vasc Surg 62:1245–50.e1. https://doi.org/10.1016/j.jvs.2015.06.136
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Francesco Vasuri: study design, data analysis, histopathological analysis, paper drafting.
Carmen Ciavarella: study design, data analysis, performed experiments, paper drafting.
Silvia Fittipaldi: study design, performed experiments.
Rodolfo Pini: angio-CT, data analysis.
Andrea Vacirca: clinical data collection and analysis.
Mauro Gargiulo: clinical data collection, paper critical revision.
Gianluca Faggioli: angio-CT, paper critical revision.
Gianandrea Pasquinelli: study design, paper critical revision, paper drafting.
All authors gave substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; gave final approval of the final version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
The present work was performed on archival tissue of human subjects, in compliance with Ethical standard. All patients are anonymous.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Vasuri, F., Ciavarella, C., Fittipaldi, S. et al. Different histological types of active intraplaque calcification underlie alternative miRNA-mRNA axes in carotid atherosclerotic disease. Virchows Arch 476, 307–316 (2020). https://doi.org/10.1007/s00428-019-02659-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02659-w