Abstract
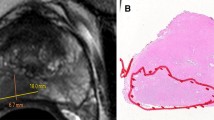
Prognostic value of tumor volume for biochemical recurrence of prostate cancer remains controversial. We aimed to determine which tumor volume definition would optimally correlate with established prognostic factors and classify macroscopic tumor configuration. Radical prostatectomy specimens with follow-up to biochemical recurrence in the period between 2009 and 2012 were retrieved. Newly proposed categories of reconstructed three-dimensional macroscopic tumor configuration were nodular, medial prominence, subcapsular spreading, and miliary types. Several algorithms were applied to identify optimal tumor volume including (1) combined volume of all nodules, (2) volume of largest nodule as index tumor, and (3) volume of nodule with strongest evidence of poor prognosis. Macroscopic typing correlated well with radiologic findings, and nodular type was most common (70.7 %). In most multifocal tumors, the largest nodule showed the highest Gleason score (90.8 %) as well as extraprostatic extension or seminal vesicle invasion (93.5 %). Total tumor and index tumor volumes were significant predictors of biochemical recurrence (both, P < 0.0001). Tumor volume, classified in three groups with cutoff values at 2 and 5 cm3, was independently predictive of recurrence-free survival in multivariate analysis (P < 0.05) and surpassed bilaterality even in stage pT2. In pT2 disease, recurrence-free survival was significantly associated with total tumor volume (P = 0.003) and index tumor volume (P = 0.002), but not with pT2 substage (P = 0.278). The proposed macroscopic classification system can be correlated with preoperative imaging findings. Total tumor or index tumor volume significantly predicts biochemical recurrence. Tumor volume classification is easy to apply in practice with high reproducibility and offsets the limitations of pT classification.


Similar content being viewed by others
References
Arora R, Koch MO, Eble JN, Ulbright TM, Li L, Cheng L (2004) Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer 100(11):2362–2366. doi:10.1002/cncr.20243
Andreoiu M, Cheng L (2010) Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol 41(6):781–793. doi:10.1016/j.humpath.2010.02.011
McNeal JE, Price HM, Redwine EA, Freiha FS, Stamey TA (1988) Stage a versus stage B adenocarcinoma of the prostate: morphological comparison and biological significance. J Urol 139(1):61–65
Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Epstein JI, Cascinu S, Briganti A, Catto JW, Montorsi F, Montironi R (2016) Current histopathologic and molecular characterisations of prostate cancer: towards individualised prognosis and therapies. Eur Urol 69(2):186–190. doi:10.1016/j.eururo.2015.05.041
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, Committee IG (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 29(9):1228–1242
van der Kwast TH, Amin MB, Billis A, Epstein JI, Griffiths D, Humphrey PA, Montironi R, Wheeler TM, Srigley JR, Egevad L, Delahunt B, Group IPC (2011) International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol 24(1):16–25. doi:10.1038/modpathol.2010.156
Ramos CG, Roehl KA, Antenor JA, Humphrey PA, Catalona WJ (2004) Percent carcinoma in prostatectomy specimen is associated with risk of recurrence after radical prostatectomy in patients with pathologically organ confined prostate cancer. J Urol 172(1):137–140. doi:10.1097/01.ju.0000132139.40964.75
Eichelberger LE, Koch MO, Eble JN, Ulbright TM, Juliar BE, Cheng L (2005) Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Mod Pathol 18(7):886–890. doi:10.1038/modpathol.3800405
Chun FK, Briganti A, Jeldres C, Gallina A, Erbersdobler A, Schlomm T, Walz J, Eichelberg C, Salomon G, Haese A, Currlin E, Ahyai SA, Benard F, Huland H, Graefen M, Karakiewicz PI (2007) Tumour volume and high grade tumour volume are the best predictors of pathologic stage and biochemical recurrence after radical prostatectomy. Eur J Cancer 43(3):536–543. doi:10.1016/j.ejca.2006.10.018
Salomon L, Levrel O, Anastasiadis AG, Irani J, De La Taille A, Saint F, Vordos D, Cicco A, Hoznek A, Chopin D, Abbou CC (2003) Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol 43(1):39–44
Porten SP, Cooperberg MR, Carroll PR (2010) The independent value of tumour volume in a contemporary cohort of men treated with radical prostatectomy for clinically localized disease. BJU Int 105(4):472–475. doi:10.1111/j.1464-410X.2009.08774.x
Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, Schroder FH, van der Kwast TH (2010) Should pathologists routinely report prostate tumour volume? The prognostic value of tumour volume in prostate cancer. Eur Urol 57(5):821–829. doi:10.1016/j.eururo.2009.07.027
Kikuchi E, Scardino PT, Wheeler TM, Slawin KM, Ohori M (2004) Is tumor volume an independent prognostic factor in clinically localized prostate cancer? J Urol 172(2):508–511. doi:10.1097/01.ju.0000130481.04082.1a
van Oort IM, Witjes JA, Kok DE, Kiemeney LA, Hulsbergen-vandeKaa CA (2008) Maximum tumor diameter is not an independent prognostic factor in high-risk localized prostate cancer. World J Urol 26(3):237–241. doi:10.1007/s00345-008-0242-7
Huang CC, Deng FM, Kong MX, Ren Q, Melamed J, Zhou M (2014) Re-evaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Archiv: an International Journal of Pathology 464(5):589–594. doi:10.1007/s00428-014-1557-y
Chen ME, Johnston DA, Tang K, Babaian RJ, Troncoso P (2000) Detailed mapping of prostate carcinoma foci: biopsy strategy implications. Cancer 89(8):1800–1809
Scattoni V, Montironi R, Mazzucchelli R, Freschi M, Nava L, Losa A, Terrone C, Scarpa RM, Montorsi F, Pappagallo G, Rigatti P (2006) Pathological changes of high-grade prostatic intraepithelial neoplasia and prostate cancer after monotherapy with bicalutamide 150 mg. BJU Int 98(1):54–58. doi:10.1111/j.1464-410X.2006.06204.x
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading C (2016) The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 40(2):244–252. doi:10.1097/PAS.0000000000000530
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Futterer JJ, European Society of Urogenital R (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22(4):746–757. doi:10.1007/s00330-011-2377-y
Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, Scheenen TW, Vos PC, Huisman H, van Oort IM, Witjes JA, Heerschap A, Futterer JJ (2011) Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261(1):46–66. doi:10.1148/radiol.11091822
Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, Choyke PL, Harisinghani M (2012) Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol 198(6):1277–1288. doi:10.2214/AJR.12.8510
Tamada T, Sone T, Jo Y, Yamamoto A, Yamashita T, Egashira N, Imai S, Fukunaga M (2008) Prostate cancer: relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology 248(2):531–539. doi:10.1148/radiol.2482070157
Kim KH, Lim SK, Shin TY, Kang DR, Han WK, Chung BH, Rha KH, Hong SJ (2013) Tumor volume adds prognostic value in patients with organ-confined prostate cancer. Ann Surg Oncol 20(9):3133–3139. doi:10.1245/s10434-013-3016-4
Hansen J, Bianchi M, Sun M, Rink M, Castiglione F, Abdollah F, Steuber T, Ahyai SA, Steurer S, Gobel C, Freschi M, Montorsi F, Shariat SF, Fisch M, Graefen M, Karakiewicz PI, Briganti A, Chun FK (2014) Percentage of high-grade tumour volume does not meaningfully improve prediction of early biochemical recurrence after radical prostatectomy compared with Gleason score. BJU Int 113(3):399–407. doi:10.1111/bju.12424
Noguchi M, Stamey TA, McNeal JE, Yemoto CE (2000) Assessment of morphometric measurements of prostate carcinoma volume. Cancer 89(5):1056–1064
Marks RA, Lin H, Koch MO, Cheng L (2007) Positive-block ratio in radical prostatectomy specimens is an independent predictor of prostate-specific antigen recurrence. Am J Surg Pathol 31(6):877–881. doi:10.1097/01.pas.0000213429.61374.4f
Eichelberger LE, Koch MO, Daggy JK, Ulbright TM, Eble JN, Cheng L (2003) Predicting tumor volume in radical prostatectomy specimens from patients with prostate cancer. Am J Clin Pathol 120(3):386–391. doi:10.1309/82U1-089X-LQGK-MMN1
Chen ME, Johnston D, Reyes AO, Soto CP, Babaian RJ, Troncoso P (2003) A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Pathol 27(10):1291–1301
Wise AM, Stamey TA, McNeal JE, Clayton JL (2002) Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 60(2):264–269
Epstein JI (2011) Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. J Urol 186(3):790–797. doi:10.1016/j.juro.2011.02.2695
Eichelberger LE, Cheng L (2004) Does pT2b prostate carcinoma exist? Critical appraisal of the 2002 TNM classification of prostate carcinoma. Cancer 100(12):2573–2576. doi:10.1002/cncr.20305
Kordan Y, Chang SS, Salem S, Cookson MS, Clark PE, Davis R, Herrell SD, Baumgartner R, Phillips S, Smith JA Jr, Barocas DA (2009) Pathological stage T2 subgroups to predict biochemical recurrence after prostatectomy. J Urol 182(5):2291–2295. doi:10.1016/j.juro.2009.07.020
Chun FK, Briganti A, Lebeau T, Fradet V, Steuber T, Walz J, Schlomm T, Eichelberg C, Haese A, Erbersdobler A, McCormack M, Perrotte P, Graefen M, Huland H, Karakiewicz PI (2006) The 2002 AJCC pT2 substages confer no prognostic information on the rate of biochemical recurrence after radical prostatectomy. Eur Urol 49(2):273–278 . doi:10.1016/j.eururo.2005.12.009discussion 278-279
Hong SK, Han BK, Chung JS, Park DS, Jeong SJ, Byun SS, Choe G, Lee SE (2008) Evaluation of pT2 subdivisions in the TNM staging system for prostate cancer. BJU Int 102(9):1092–1096. doi:10.1111/j.1464-410X.2008.07897.x
Freedland SJ, Partin AW, Epstein JI, Walsh PC (2004) Biochemical failure after radical prostatectomy in men with pathologic organ-confined disease: pT2a versus pT2b. Cancer 100(8):1646–1649. doi:10.1002/cncr.20145
Acknowledgments
This study was supported by the Korean Foundation for Cancer Research and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI13C0858).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board of Severance Hospital (4–2013-0857).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Su-Jin Shin and Cheol Keun Park contributed equally to this work.
Electronic supplementary material
Table S1
(DOCX 20 kb)
Table S2
(DOCX 17 kb)
Table S3
(DOCX 18 kb)
Table S4
(DOCX 21 kb)
Table S5
(DOCX 20 kb)
Supplementary Fig. S1
Representative macroscopic configuration of multifocal prostate tumors. Representative macroscopic configuration of multifocal tumors with 1:1 scan images with H&E staining of coronal whole-mount sections of radical prostatectomy specimens. Circles represent cancer nodules. In multifocal disease, the largest nodule usually showed the worst Gleason score (a) and extraprostatic extension (EPE) or seminal vesicle invasion (SVI) (b). However, in some cases, the highest Gleason score (c) and EPE/SVI (d) were not associated with the largest nodule. (GIF 321 kb)
Supplementary Fig. S2
Comparison of biochemical recurrence (BCR)-free survival rates after radical prostatectomy according to macroscopic tumor configuration. BCR-free survival rates were low in patients with medial prominence/subcapsular spread types than nodular/military types. (GIF 28 kb)
Supplementary Fig. S3
Receiver operating characteristic (ROC) and area under the curve (AUC) for tumor volumes (TVs). (GIF 29 kb)
Rights and permissions
About this article
Cite this article
Shin, SJ., Park, C.K., Park, S.Y. et al. Total intraglandular and index tumor volumes predict biochemical recurrence in prostate cancer. Virchows Arch 469, 305–312 (2016). https://doi.org/10.1007/s00428-016-1971-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1971-4