Abstract
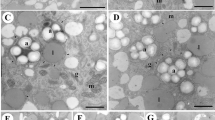
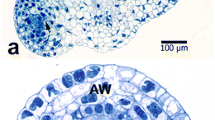
Vacuoles of several types can be observed in pollen throughout its development. Their physiological significance reflects the complexity of the biological process leading to functional pollen grains. Vacuolisation always occurs during pollen development but when ripe pollen is shed the extensive translucent vacuoles present in the vegetative parts in previous stages are absent. Vacuole functions vary according to developmental stage but in ripe pollen they are mainly storage sites for reserves. Vacuoles cause pollen to increase in size by water accumulation and therefore confer some degree of resistance to water stress. Modalities of vacuolisation occur in pollen in the same manner as in other tissues. In most cases, autophagic vacuoles degrade organelles, as in the microspore after meiosis, and can be regarded as cytoplasm clean-up following the transition from the diploid sporophytic to the haploid gametophytic state. This also occurs in the generative cell but not in sperm cells. Finally, vacuoles have a function when microspores are used for pollen embryogenesis in biotechnology being targets for stress induction and afterwards contributing to cytoplasmic rearrangement in competent microspores.







Similar content being viewed by others
References
Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59:469–484
Aouali N, Laporte P, Clément C (2001) Pectin secretion and distribution in the anther during pollen development in Lilium. Planta 213:71–79
Baldi BG, Franceschi VR, Loewus FA (1987) Localization of phosphorus and cation reserves in Lilium longiflorum pollen. Plant Physiol 83:1018–1021
Baskin CC, Baskin JM (1998) Seeds: ecology biogeography and evolution of dormancy and germination. Academic Press, San Diego
Benito-Moreno RM, Macke F, Hause MT, Alwen A, Heberle-Bors E (1988) Sporophytes and male gametophytes from in vitro cultured, immature tobacco pollen. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin, pp 137–142
Blackmore S, Barnes S (1990) Pollen wall development in angiosperms. In: Blackmore S, Knox RB (eds) Microspore: evolution and ontogeny. Academic Press, London, pp 173–192
Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140:1151–1168
Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot 60:1465–1478
Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005) PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J Exp Bot 56:113–121
Brown RC, Lemmon BE (1981) Aperture development in spore of the moss, Trematodon longicollis Mx. Protoplasma 106:273–287
Butow R, Rodriguez-Garcia MI, Alché JD, Gorska-Brylass A (1997) Calcium in electron-dense globoids during pollen grain maturation in Chlorophytum elatum R.Br. Planta 203:413–421
Cass DD, Karas I (1975) Development of sperm cells in barley. Can J Bot 53:1051–1062
Chang T, Neuffer MG (1989) Maize microsporogenesis. Genome 32:232–244
Christensen JE, Horner HT (1974) Pollen pore development and its spatial orientation during microsporogenesis in the grass Sorghum bicolor. Am J Bot 61:604–623
Clément C, Audran JC (1996) In vivo and in vitro pollen maturation of Lilium. Influence of carbohydrates. Acta Soc Bot Pol 65:73–82
Clément C, Pacini E (2001) Anther plastids in angiosperms. Bot Rev 67:54–73
Clément C, Chavant L, Burrus M, Audran JC (1994) Anther starch variations in Lilium during pollen development. Sex Plant Reprod 7:347–356
Clément C, Laporte P, Audran JC (1998) The loculus content and tapetum during pollen development in Lilium. Sex Plant Reprod 11:94–106
Clément C, Sangwan RS, Sangwan BS (2005) Microspore embryo induction and development in higher plants: cytological and ultrastructural aspects. In: Palmer CE, Keller WA, Kasha KJ (eds) Haploids in crop improvement II. Springer, Berlin, pp 53–72
Cran DR (1979) The ultrastructure of fern gametophyte cells. In: Dyer AF (ed) The experimental biology of ferns. Academic Press, London, pp 171–213
Dai S, Li L, Chen T, Chong K, Xue Y, Wang T (2006) Proteomic analyses of Oryza sativa mature pollen reveal novel proteins associated with pollen germination and tube growth. Proteomics 6:2504–2529
Dettmer J, Schubert D, Calvo-Weimar O, Stierhof Y-D, Schmidt R, Schumacher K (2005) Essential role of V-ATPase in male gametophyte development. Plant J 41:117–124
Dottge JD (1973) The fine structure of algal cells. Academic Press, London
Evans DE, Taylor PE, Singh MB, Knox RB (1991) Quantitative analysis of lipids and protein from the pollen of Brassica napus L. Plant Sci 73:117–126
Evans DE, Taylor PE, Singh MB, Knox RB (1992) The interrelationship between the accumulation of lipids, protein and level of acyl carrier protein during development of Brassica napus L. pollen. Planta 186:343–354
Footitt S, Cohn MA (2001) Developmental arrest: from sea urchin to seeds. Seed Sci Res 11:2–16
Franchi GG, Bellani LM, Nepi M, Pacini E (1996) Types of carbohydrate reserves in pollen: localization, systematic distribution and ecophysiological significance. Flora 191:143–159
Franchi GG, Nepi M, Pacini E (2002) Partially hydrated pollen: taxonomic distribution, ecological and evolutionary significance. Plant Syst Evol 234:211–227
Garrido D, Vicente O, Heberle-Bors E, Rodriguez-Garcia I (1995) Cellular changes during the acquisition of embryogenic potential in isolated pollen grains of Nicotiana tabacum. Protoplasma 186:220–230
Helsper JPFG, Linskens HF, Jackson JF (1984) Phytate metabolism in Petunia pollen. Phytochemistry 23:1841–1845
Heslop-Harrison J (1979) An interpretation of the hydrodynamics of pollen. Am J Bot 66:737–743
Heslop-Harrison J (1987) Pollen germination and pollen-tube growth. Int Rev Cytol 107:1–78
Heslop-Harrison J, Heslop-Harrison Y, Heslop-Harrison JS (1997) Motility in ungerminated grass pollen: association of myosin with polysaccharide-containing wall-precursors bodies (P bodies). Sex Plant Reprod 10:65–66
Hicks GR, Rojo E, Hong S, Carter DG, Raikhel N (2004) Germinating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol 134:1227–1239
Hoekstra FA, van Zijderweld MH, Heidekamp F, van der Mark F (1993) Microspore culture of Hordeum vulgare L.: the influence of density and osmolality. Plant Cell Rep 12:661–665
Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5:4864–4884
Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132:640–652
Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D (1998) Selection of T-DNA tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics 149:621–631
Ikeda S, Nasrallah JB, Dixit R, Preiss S, Nasrallah ME (1997) An aquaporin-like gene required for the Brassica self-incompatibility response. Science 276:1564–1566
Indrianto A, Barinova I, Touraev A, Heberle-Bors E (2001) Tracking individual wheat microspores in vitro: identification of embryogenic microspores and body axis formation in the embryo. Planta 212:163–174
Jacquard C, Mazeyrat-Gourbeyre F, Devaux P, Boutilier K, Baillieul F, Clément C (2009) Microspore embryogenesis in barley: anther pre-treatment stimulates plant defence gene expression. Planta 229:393–402
Jakobsen MK, Poulsen LR, Schulz A, Fleurat-Lessard P, Moller A, Husted S, Schiott M, Amtmann A, Palmgren MG (2005) Pollen development and fertilization in Arabidopsis is dependent on the male gametogenesis impaired anthers gene encoding a type V P-type ATPase. Genes Dev 19:2757–2769
John P (1991) Fructan quality and fructan synthesis. Biochem Soc T 19:569–572
Kjellbom P, Larsson C, Johansson I, Karlsson M, Johanson U (1999) Aquaporins and water homeostasis in plants. Trends Plant Sci 4:308–314
Knox RB, Ducker SC (1991) The evolution of gametes—from motility to double fertilization. In: Blackmore S, Barnes SH (eds) Pollen and spores-pattern of diversification. Systematic Association Special Volume n°44. Clarendon Press, Oxford, pp 345–361
Kuang A, Musgrave ME (1996) Dynamics of vegetative cytoplasm during generative cell formation and pollen maturation in Arabidopsis thaliana. Protoplasma 194:81–90
Kumlehn J, Lörz H (1999) Monitoing sporophytic development of individual microspores of barley (Hordeum vulgare L.). In: Clément C, Pacini E, Audran JC (eds) Anther and pollen: from biology to biotechnology. Springer, Berlin, pp 183–190
Luu D-T, Maurel C (2005) Acquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ 28:85–96
Maeshima M, Ishikawa F (2008) ER membrane aquaporins in plants. Eur J Physiol 456:709–716
Maraschin SF, Vennik M, Lamers GEM, Spaink HP, Wang M (2005) Time-lapse tracking of barley androgenesis reveals position-determined cell death within pro-embryos. Planta 220:531–540
Margulis L, Schwartz KV (1982) Five Kingdoms: an illustrated guide to the phyla of life on earth. Freeman and Co, San Francisco
Marty F (1999) Plant vacuoles. Plant Cell 11:587–599
Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 48:399–429
McConchie CA, Knox RB (1989) Pollination and reproductive biology of seagrasses. In: Larkum AWD, McComb AJ, Sheperd SA (eds) Biology of seagrasses. Elsevier, Amsterdam, pp 74–111
McConchie CA, Hough T, Knox RB (1987) Ultrastructural analysis of the sperm cells of mature pollen of maize, Zea mays. Protoplasma 139:9–19
McCormick S (2004) Control of male gametophyte development. Plant Cell 16:142–153
McCormick S, Curie C, Eyal Y, Muschietti J, Dirks L, Kulikauskas R (1994) Molecular biology of male gametogenesis. Euphytica 79:245–250
Mckenzie A, Heslop-Harrison J, Dickinson HG (1967) Elimination of ribosomes during meiotic prophase. Nature 215:997–999
Mimura T, Kura-Hotta M, Tsujimura T, Ohnishi M, Miura M, Okazaki Y, Miura M, Maeshima M, Washitani-Nemoto S (2003) Rapid increase of vacuolar volume in response to salt stress. Planta 216:397–402
Nagata N, Saito C, Sakai A, Kuroiwa H, Kuroiwa T (2000) Unique positioning of mitochondria in developing microspores and pollen grains in Pharbitis nil: mitochondria cover the nuclear surface at specific developmental stages. Protoplasma 213:74–82
Neidhart HV (1979) Comparative studies of sporogenesis in Bryophytes. In: Clarke CGS, Duckett JS (eds) Bryophyte systematics. Academic Press, London, pp 251–280
Nepi M, Franchi GG, Pacini E (2001) Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma 216:171–180
Niewiadomski P, Knappe S, Geimer S, Fischer K, Schulz B, Unte US, Rosso MG, Ache P, Flügge U-F, Schneider A (2005) The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 17:746–759
O’Brien M, Bertrand C, Matton DP (2002) Characterization of a fertilization-induced and developmentally regulated plasma-membrane aquaporin expressed in reproductive tissues, in the wild potato Solanum chacoense Bitt. Planta 215:485–493
Oldenhof MT, Groot PFM, Visser JH, Schrauven JAM, Wullems GJ (1996) Isolation and characterization of a microspore-specific gene from tobacco. Plant Mol Biol 31:213–225
Pacini E (1996) Types and meaning of pollen carbohydrates reserves. Sex Plant Reprod 9:362–366
Pacini E (2000) From anther and pollen ripening to pollen presentation. Plant Syst Evol 22:19–43
Pacini E, Franchi GG (1983) Pollen grain development in Smilax aspera and possible function of the loculus. In: Mulcahy DL, Ottaviano E (eds) Pollen: biology and implications in plant breeding. Elsevier Biomedical, New York, pp 183–190
Pacini E, Hesse M (2002) Types of pollen dispersal units in orchids, and their consequences for germination and fertilisation. Ann Bot 89:653–664
Pacini E, Hesse M (2004) Cytophysiology of pollen presentation and dispersal. Flora 199:273–285
Pacini E, Juniper BJ (1984) The ultrastructure of pollen grain development in Lycopersicum peruvianum. Caryologia 37:21–50
Pacini E, Keijzer CJ (1989) Ontogeny of intruding non periplasmodial tapetum in the wild chicory (Cichorium intybus L. Compositae). Plant Syst Evol 167:149–164
Pacini E, Bellani LM, Lozzi R (1986) Pollen, tapetum and anther development in two cultivars of sweet cherry (Prunus avium). Pytomorphology 36:197–210
Pacini E, Taylor PE, Singh MB, Knox RB (1992) Development of plastids, including amyloplasts and starch grains, in pollen and tapetum of rye-grass, Lolium perenne. Ann Bot 70:179–188
Pandolfi T, Pacini E, Calder DM (1993) Ontogenesis of monad pollen in Pterostylis plumosa (Orchidaceae, Neottioideae). Plant Syst Evol 186:175–185
Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434:404–408
Pertl H, Schulze WX, Obermeyer G (2009) The pollen organelle membrane proteome reveals highly spatial-temporal dynamics during germination and tube growth of lily pollen. J Proteome Res 8:5142–5152
Reisen D, Marty M, Leborgne-Castel N (2005) New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamics during osmotic stress. BMC Plant Biol 5:1–13
Rojo E, Zouhar J, Kovaleva V, Hong S, Raikhel NV (2003) The AtC-VPS protein complex is localized to the tonoplast and the prevacuolar compartment in Arabidopsis. Mol Biol Cell 14:361–369
Ruiter RK, van Eldik GJ, van Herpen MMA, Schrauwen JM, Wullems GJ (1997) Expression in anthers of two genes encoding Brassica oleracea transmembrane channel proteins. Plant Mol Biol 34:163–168
Russell SD (1984) Ultrastructure of the sperm of Plumbago zeylanica: II. Quantitative cytology and three-dimensional organization. Planta 162:385–391
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Sangwan RS (1986) Formation and cytochemistry of nuclear vacuoles during meiosis in Datura. Eur J Cell Biol 40:210–218
Sangwan RS, Camefort H (1983) The tonoplast, a specific marker of embryogenic microspores of Datura cultured in vitro. Histochemistry 78:473–480
Sangwan RS, Sangwan-Norreel BS (1987) Ultrastructural cytology of plastids in pollen grains of certain androgenic and nonandrogenic plants. Protoplasma 138:11–22
Sangwan RS, Mathivet V, Vasseur G (1989) Ultrastructural localization of acid phosphatase during male meiosis and sporogenesis in Datura: evidence for digestion of cytoplasmic structures in the vacuoles. Protoplasma 149:38–46
Schlag M, Hesse M (1992) The formation of the generative cell in Polystachya pubescens (Orchidaceae). Sex Plant Reprod 5:131–137
Sheoran IS, Pedersen EJ, Ross ARS, Sawhney VK (2009) Dynamics of protein expression during pollen germination in canola (Brassica napus). Planta 230:779–793
Soto G, Alleva K, Mazzella MA, Amodeo G, Muschiett JP (2008) AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett 582:4077–4082
Southworth D, Dickinson DB (1981) Ultrastructural changes in germinating lily pollen. Grana 20:29–35
Speranza A, Calzoni GL, Pacini E (1997) Occurrence of mono- or disaccharides and polysaccharide reserves in mature pollen grains. Sex Plant Reprod 10:110–115
Strompen G, Dettmer J, Stierhof Y-D, Schumacher K, Jürgens G, Mayer U (2005) Arabidopsis H+-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J 41:125–132
Theunis CH, Cresti M, Milanesi C (1991) Studies of the mature pollen of Spinacia oleracea after freeze substitution and observed with confocal laser scanning fluorescence microscopy. Bot Acta 104:324–329
Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:297–302
Twell D, Ki Park S, Lalanne E (1998) Asymmetric division and cell-fate determination in developing pollen. Trends Plant Sci 3:305–310
Weber M (1988) Metabolism of P-particles (polysaccharide particles) in mature pollen grains of Eryngium campestre L. (Apiaceae). Protoplasma 146:65–71
Wilson C, Voronin V, Touraev A, Vicente O, Heberle-Bors E (1997) A developmentally regulated MAP kinase activated by hydration in tobacco pollen. Plant Cell 9:2093–2100
Wojnarowiez G, Caredda S, Devaux P, Sangwan RS, Clément C (2004) Effects of different osmotica on androgenesis and albinism in barley (Hordeum vulgare L.). J Plant Physiol 161:747–755
Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T (2003) Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44:1192–1201
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacini, E., Jacquard, C. & Clément, C. Pollen vacuoles and their significance. Planta 234, 217–227 (2011). https://doi.org/10.1007/s00425-011-1462-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1462-4