Abstract
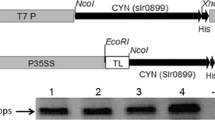
Cassava (Manihot esculenta Crantz.) is the major source of calories for subsistence farmers in sub-Saharan Africa. Cassava, however, contains potentially toxic levels of the cyanogenic glucoside, linamarin. The cyanogen content of cassava foods can be reduced to safe levels by maceration, soaking, rinsing and baking; however, short-cut processing techniques can yield toxic food products. Our objective was to eliminate cyanogens from cassava so as to eliminate the need for food processing. To achieve this goal we generated transgenic acyanogenic cassava plants in which the expression of the cytochrome P450 genes (CYP79D1 and CYP79D2), that catalyze the first-dedicated step in linamarin synthesis, was inhibited. Using a leaf-specific promoter to drive the antisense expression of the CYP79D1/CYP79D2 genes we observed up to a 94% reduction in leaf linamarin content associated with an inhibition of CYP79D1 and CYP79D2 expression. Importantly, the linamarin content of roots also was reduced by 99% in transgenic plants having between 60 and 94% reduction in leaf linamarin content. Analysis of CYP79D1/CYP79D2 transcript levels in transgenic roots indicated they were unchanged relative to wild-type plants. These results suggest that linamarin is transported from leaves to roots and that a threshold level of leaf linamarin production is required for transport.





Similar content being viewed by others
References
Anderssen M, Bush P, Svendsen I, Moller B (2000) Cytochromes P450 from cassava catalyzing the first steps in the biosynthesis of the cyanogenic glycosides linamarin and lotaustralin. J Biol Chem 275:1966–1975
Arias-Garzon D, Sayre R (1993) Tissue specific inhibition of transient gene expression in cassava (Manihot esculenta Crantz). Plant Sci 93:121–130
Arias-Garzon D, Sayre R (2000) Genetic engineering approaches to reducing the cyanide toxicity in cassava (Manihot esculenta, Crantz). In: Carvalho L, Thro AM, Vilarinhos AD (eds) Proceedings of the fourth international scientific meeting of the Cassava Biotechnology Network, Embrapa, Brasilia, pp 213–221
Arias-Garzon D, Sarria R, Gelvin S, Sayre R (1994) New Agrobacterium tumefaciens plasmids for cassava transformation. In: Roca WM, Thro AM (eds) Proceedings of the second international scientific meeting of the Cassava Biotechnology Network, Bogor, Indonesia, pp 245–256
Bediako M, Tapper B, Pritchard G (1981) Metabolism synthetic site and translocation of cyanogenic glucoside in cassava. In: Terry ER (ed) Proceedings of the first triennial root crops symposium of the International Society for Tropical Root Crops. IDRC, Canada, pp 143–148
Best R, Hargrove T (1994) Casssava: the latest facts about an ancient crop. CIAT Publication, Cali, Colombia
Bevan W, Barnes W, Chilton M (1983) Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res 11:369–385
Brusslan J, Tobin E (1992) Light-independent developmental regulation of cab gene expression in Arabidopsis thaliana seedlings. Proc Natl Acad Sci USA 89:7791–7795
Doyle J, Doyle J (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Du L, Bokanga M, Moller B, Halkier B (1995) The biosynthesis of cyanogenic glucosides in roots of cassava. Phytochemistry 39:323–326
Gamborg O, Miller R, Ojima K (1968) Nutrient requirement of suspension cultures of soybean and carrot cells. Exp Cell Res 50:151–158
Hughes J, Carvalho F, Hughes M (1994) Purification, characterization and cloning of hydroxynitrile lyase from cassava (Manihot esculenta Crantz). Arch Biochem Biophys 311:496–502
Ikoigbo B (1980) Nutritional implications of projects giving high priority to the production of staples of low nutritive quality. The case of cassava in the humid tropics of West Africa. Food and Nutrition Bulletin, United Nations University, Tokyo, vol 2, pp 1–10
Kahn R, Bak S, Svendsen I, Halkier B, Moller B (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol 115:1661–1670
Kakes P (1990) Properties and functions of the cyanogenic system in higher plants. Euphytica 48:25–43
Koch B, Nielsen V, Halkier B, Olsen C, Møller B (1992) The biosynthesis of cyanogenic glycosides in seedlings of cassava (Manihot esculenta Crantz). Arch Biochem Biophys 292:141–150
Latham M (1979) Human nutrition in tropical Africa. Food and Agriculture Organization of the United Nations, Rome
Li H-Q, Sautter C, Potrykus I, Puonti-Kaerlas J (1996) Genetic transformation of cassava (Manihot esculenta Crantz). Nature Biotechnol 14:736–740
Makame M, Akoroda M, Hahn S (1987) Effects of reciprocal stem grafts on cyanide translocation in cassava. J Agric Sci Cambridge 109:605–608
McMahon J, Sayre R (1994) Regulation of cyanogenic potential in cassava (Manihot esculenta Crantz). In: Roca WM, Thro AM (eds) Proceedings of the second international scientific meeting of the Cassava Biotechnology Network, Bogor, Indonesia, pp 423–438
McMahon J, White W, Sayre R (1995) Cyanogenesis in cassava (Manihot esculenta Crantz). J Exp Bot 46:731–741
Mkpong O, Yan H, Chism G, Sayre R (1990) Purification, characterization, and localization of linamarase in cassava. Plant Physiol 93:176–181
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15:473–497
Osuntokun B (1991) Cassava diet, chronic cyanide intoxification and neuropathy in Nigerian Africans. World Rev Nutr Diet 36:141–173
Ramanujam T, Indira P (1984) Effect of girdling on the distribution of total carbohydrates and hydrocyanic acid in cassava. Indian J Plant Phys 27:355–360
Rosling H (1974) Cassava cyanide and epidemic spastic paraparesis: a study in Mozambique on dietery cyanide exposure. ACTA Universitatis Upsalienis, Ph.D. thesis, Uppsala University, Sweden
Rosling H, Mlingi N, Tylleskar T, Banea M (1992) Causal mechanisms behind human diseases induced by cyanide exposure from cassava. In: Roca WM, Thro AM (eds) Proceedings of the first international scientific meeting of the Cassava Biotechnology Network, Cartegena, Cali, Colombia, pp 366–375
Sambrook J, Fritsch E, Maniatis J (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Selmar D (1993) Transport of cyanogenic glucosides: linustatin uptake in Hevea cotyledons. Planta 191:191–199
Selmar D, Lieberei R, Biehl R (1988) Mobilization and utilization of cyanogenic glycosides: the linustatin pathway. Plant Physiol 86:711–716
Sibbensen O, Koch B, Halkier B, Moller B (1994) Isolation of a heme-thiolate enzyme cytochrome P450TYR which catalyzes the committed step in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Proc Natl Acad Sci USA 91:9740–9744
Soni R, Murray A (1994) Isolation of intact DNA and RNA from plant tissue. Anal Biochem 218:474–476
Stitt M, Sonnewald U (1995) Regulation of metabolism in transgenic plants. Annu Rev Plant Physiol Plant Mol Biol 46:341–368
Tylleskar T, Banea M, Bikangi N, Cooke R, Poulter N, Rosling H (1992) Cassava cyanogens and konzo, an upper motor neuron disease found in Africa. Lancet 339:208–211
Vincent J (1985) A manual for the practical study of root-nodule bacteria. IBP handbook No. 15
Wheatley C, Orrego J, Sanchez T, Granados E (1993) Quality evaluation of cassava core collection at CIAT. In: Roca WM, Thro AM (eds) Proceedings of the first international scientific meeting of the Cassava Biotechnology Network, Cartegena, Cali, Colombia, pp 255–264
White W, McMahon J, Sayre R (1994) Regulation of cyanogenesis in cassava. Acta Hort 375:69–77
White W, Arias-Garzon D, McMahon J, Sayre R (1998) Cyanogenesis in cassava: the role of hydroxynitrile lyase in root cyanide production. Plant Physiol 116:1219–1225
Acknowledgements
Special thanks to Dr. Johnnie Brown of the Ohio State University, Campus Chemical Instrument Center for GC–MS method development for linamarin quantification and Dr. J.C. Jang of the Ohio State University, Horticulture and Crop Science department for critical comments on this manuscript. This research has been supported by grants from the Rockefeller Foundation, the Consortium for Plant Biotechnology Research, the Cassava Biotechnology Network, and the Ohio State University (to R.T.S.). This paper is dedicated to Dr. Chusa Ginés and Verónica Mera.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siritunga, D., Sayre, R.T. Generation of cyanogen-free transgenic cassava. Planta 217, 367–373 (2003). https://doi.org/10.1007/s00425-003-1005-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1005-8