Abstract
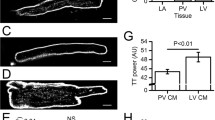
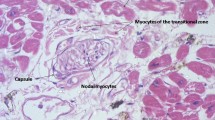
The electrophysiological properties of the superior vena cava (SVC) myocardium, which is considered a minor source of atrial arrhythmias, were studied in this study during postnatal development. Conduction properties were investigated in spontaneously active and electrically paced SVC preparations obtained from 7–60-day-old male Wistar rats using optical mapping and microelectrode techniques. The presence of high-conductance connexin 43 (Cx43) was evaluated in SVC cross-sections using immunofluorescence. It was found that SVC myocardium is excitable, electrically coupled with the atrial tissue, and conducts excitation waves at all stages of postnatal development. However, the conduction velocity (CV) of excitation and action potential (AP) upstroke velocity in SVC were significantly lower in neonatal than in adult animals and increased with postnatal maturation. Connexins Cx43 were identified in both neonatal and adult rat SVC myocardium; however, the abundance of Cx43 was significantly less in neonates. The gap junction uncoupler octanol affected conduction more profound in the neonatal than in adult SVC. We demonstrated for the first time that the conduction characteristics of SVC myocardium change from a slow-conduction (nodal) to a high-conduction (working) phenotype during postnatal ontogenesis. An age-related CV increase may occur due to changes of AP characteristics, electrical coupling, and Cx43 presence in SVC cardiomyocyte membranes. Observed changes may contribute to the low proarrhythmicity of adult caval vein cardiac tissue, while pre- or postnatal developmental abnormalities that delay the establishment of the working conduction phenotype may facilitate SVC proarrhythmia.





Similar content being viewed by others
Abbreviations
- AF:
-
Atrial fibrillation
- AP:
-
action potential
- CV:
-
conduction velocity
- Cx43:
-
Connexin 43
- dv/dtmax :
-
Maximum upstroke velocity of action potential
- SAN:
-
Sinoatrial node
- SVC:
-
Superior vena cava
- SV:
-
Sinus venosus
- PV:
-
Pulmonary vein
- RA:
-
Right atrium
- RMP:
-
Resting membrane potential
References
Anderson RH, Brown NA, Moorman AFM (2006) Development and structures of the venous pole of the heart. Dev Dyn 235:2–9. https://doi.org/10.1002/dvdy.20578
Atkinson AJ, Logantha SJRJ, Hao G, Yanni J, Fedorenko O, Sinha A, Gilbert SH, Benson AP, Buckley DL, Anderson RH, Boyett MR, Dobrzynski H (2013) Functional, anatomical, and molecular investigation of the cardiac conduction system and arrhythmogenic atrioventricular ring tissue in the rat heart. J Am Heart Assoc 2:e000246. https://doi.org/10.1161/JAHA.113.000246
Boyett MR, Inada S, Yoo S, Li J, Liu J, Tellez J, Greener ID, Honjo H, Billeter R, Lei M, Zhang H, Efimov IR, Dobrzynski H (2006) Connexins in the sinoatrial and atrioventricular nodes. Adv Cardiol 42:175–197
Bruzzone R, Giaume C, Rozental R, Srinivas M, Spray DC (2003) How to close a gap junction channel: efficacies and potencies of uncoupling agents. Connexin Methods Protoc 154:447–476. https://doi.org/10.1385/1-59259-043-8:447
Bukauskas FF, Elfgang C, Willecke K, Weingart R (1995) Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J 68:2289–2298. https://doi.org/10.1016/0002-9149(58)90270-4
Camelliti P, Green CR, LeGrice I, Kohl P (2004) Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res 94:828–835. https://doi.org/10.1161/01.RES.0000122382.19400.14
Carrow R, Calhoun ML (1964) The extent of cardiac muscle in the great veins of the dog. Anat Rec 150:249–256. https://doi.org/10.1002/ar.1091500306
Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H (2009) Molecular architecture of the human sinus node insights into the function of the cardiac pacemaker. Circulation 119:1562–1575. https://doi.org/10.1161/CIRCULATIONAHA.108.804369
Christoffels VM, Mommersteeg MTM, Trowe MO, Prall OWJ, De Gier-De Vries C, Soufan AT, Bussen M, Schuster-Gossler K, Harvey RP, Moorman AFM, Kispert A (2006) Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ Res 98:1555–1563. https://doi.org/10.1161/01.RES.0000227571.84189.65
Cohen CJ, Bean BP, Tsien RW (1984) Maximal upstroke velocity as an index of available sodium conductance comparison of maximal upstroke velocity and voltage clamp measurements of sodium current in rabbit purkinje fibers. Circ Res 54:636–651. https://doi.org/10.1161/01.RES.54.6.636
Cullinan V, Campbell JH, Mosse PR, Campbell GR (1986) The morphology and cell culture of the striated musculature of the rat azygos vein. Cell Tissue Res 243:185–191. https://doi.org/10.1007/BF00221867
Diez U, Schwartze H (1991) Quantitative electrocardiography and vectorcardiography in postnatally developing rats. J Electrocardiol 24:53–62
Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, Wright SE, Parson SH, Jones SA, Lancaster MK, Yamamoto M, Honjo H, Takagishi Y, Kodama I, Efimov IR, Billeter R, Boyett MR (2005) Computer three-dimensional reconstruction of the sinoatrial node. Circulation 111:846–854. https://doi.org/10.1161/01.CIR.0000152100.04087.DB
Dobrzynski H, Boyett MR, Anderson RH (2007) New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation 115:1921–1932. https://doi.org/10.1161/CIRCULATIONAHA.106.616011
Dobrzynski H, Anderson RH, Atkinson A, Borbas Z, D’Souza A, Fraser JF, Inada S, Logantha SJRJ, Monfredi O, Morris GM, Moorman AFM, Nikolaidou T, Schneider H, Szuts V, Temple IP, Yanni J, Boyett MR (2013) Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol Ther 139:260–288. https://doi.org/10.1016/j.pharmthera.2013.04.010
Endo H, Mifune H, Kurohmaru M, Hayashi Y (1996) Cardiac musculature of the cranial vena cava in the rat. Cells Tissues Organs 151:107–111. https://doi.org/10.1159/000147650
Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, De Tombe PP (2008) Blebbistatin: Use as inhibitor of muscle contraction. Pflugers Arch - Eur J Physiol 455:995–1005. https://doi.org/10.1007/s00424-007-0375-3
Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR (2007) Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4:619–626. https://doi.org/10.1016/j.hrthm.2006.12.047
Friedman WF, Pool PE, Jacobowitz D, Seagren SC, Braunwald E (1968) Sympathetic innervation of the developing rabbit heart. Circ Res 23:25–32
Gourdie RG, Green CR, Severs NJ, Thompson RP (1992) Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anat Embryol (Berl) 185:363–378. https://doi.org/10.1007/BF00188548
Gussenhoven WJ, Essed CE, Bos E (1982) Persistent right sinus venosus valve. Br Heart J 47:183–185
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J (1998) Spontaneous initiation of atrial fibrillation by ectopi c beats originating in the pulmonary veins. N Engl J Med 339:659–666. https://doi.org/10.1056/NEJM199809033391003
Hasan W (2013) Autonomic cardiac innervation. Organogenesis 9:176–193. https://doi.org/10.4161/org.24892
Hew KW, Keller KA (2003) Postnatal anatomical and functional development of the heart: a species comparison. Birth Defects Res (Part B) 68:309–320. https://doi.org/10.1002/bdrb.10034
Hseu S-S, Yien H-W, Du F, Sun LS (1998) Heart rate variability in neonatal rats after perinatal cocaine exposure. Neurotoxicol Teratol 20:601–605. https://doi.org/10.1016/S0892-0362(98)00026-9
Jensen B, Boukens B, Wang T, Moorman A, Christoffels V (2014) Evolution of the sinus venosus from fish to human. J Cardiovasc Dev Dis 1:14–28. https://doi.org/10.3390/jcdd1010014
Jensen CF, Bartels ED, Braunstein TH, Nielsen LB, Holstein-Rathlou NH, Axelsen LN, Nielsen MS (2019) Acute intramyocardial lipid accumulation in rats does not slow cardiac conduction per se. Physiol Rep 7:e14049. https://doi.org/10.14814/phy2.14049
Jiménez-López J, Vallès E, Benito B, Martí-Almor J (2017) Insights of the superior vena cava conduction properties: a 3-D high resolution mapping case of typical flutter. J Cardiovasc Electrophysiol 29:2–3. https://doi.org/10.1111/jce.13350
Kanagaratnam P, Rothery S, Patel P, Severs NJ, Peters NS (2002) Relative expression of immunolocalized connexins 40 and 43 correlates with human atrial conduction properties. J Am Coll Cardiol 39:116–123. https://doi.org/10.1016/S0735-1097(01)01710-7
Kreuzberg MM, Willecke K, Bukauskas FF (2006) Connexin-mediated cardiac impulse propagation: connexin 30.2 slows atrioventricular conduction in mouse heart. Trends Cardiovasc Med 16:266–272. https://doi.org/10.1016/j.tcm.2006.05.002.Connexin-Mediated
Krishnan A, Samtani R, Dhanantwari P, Lee E, Yamada S, Shiota K, Donofrio MT, Leatherbury L, Lo CW (2014) A detailed comparison of mouse and human cardiac development. Pediatr Res 76:500–507. https://doi.org/10.1038/pr.2014.128
Kucera JP, Rohr S, Rudy Y (2002) Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res 91:1176–1182
Kugler S, Nagy N, Rácz G, Tőkés AM, Dorogi B, Nemeskéri Á (2018) Presence of cardiomyocytes exhibiting Purkinje-type morphology and prominent connexin45 immunoreactivity in the myocardial sleeves of cardiac veins. Heart Rhythm 15:258–264. https://doi.org/10.1016/j.hrthm.2017.09.044
Kuzmin VS, Egorov YV, Karimova VM, Rosenshtraukh ALV (2015) Evaluation of the length constant in the atrial myocardium and pulmonary vein myocardium in mammals. Dokl Biol Sci 460:8–11. https://doi.org/10.1134/S0012496615010093
Kwong KF, Schuessler RB, Green KG, Laing JG, Eric C, Boineau JP, Saffitz JE, Beyer EC (1998) Differential expression of gap junction proteins in the canine sinus node. Circ Res 82:604–613
Lee TM, Lin SZ, Chang NC (2013) Both PKA and Epac pathways mediate N-acetylcysteine-induced connexin43 preservation in rats with myocardial infarction. PLoS One 8:e71878. https://doi.org/10.1371/journal.pone.0071878
Lipp JAM, Rudolph AM (1972) Sympathetic nerve development in the rat and guinea-pig heart. Biol Neononate 21:76–82
Maier SKG, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA (2002) An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. PNAS 99:4073–4078
Marvin WJ, Hermsmeyer K, McDonald RI, Roskoski LM, Roskoski R (1980) Ontogenesis of cholinergic innervation in the rat heart. Circ Res 46:690–695. https://doi.org/10.1161/01.RES.46.5.690
Masani F (1986) Node-like cells in the myocardial layer of the pulmonary vein of rats: an ultrastructural study. J Anat 145:133–142
Miyazaki S, Takigawa M, Kusa S, Kuwahara T, Taniguchi H, Okubo K, Nakamura H, Hachiya H, Hirao K, Takahashi A, Iesaka Y (2014) Role of arrhythmogenic superior vena cava on atrial fibrillation. J Cardiovasc Electrophysiol 25:380–386. https://doi.org/10.1111/jce.12342
Momma K, Linde LM (1969) Abnormal rhythms associated with persistent left superior vena cava. Pediatr Res 3:210–216. https://doi.org/10.1203/00006450-196905000-00004
Mommersteeg MTM, Hoogaars WMH, Prall OWJ, De Gier-De Vries C, Wiese C, Clout DEW, Papaioannou VE, Brown NA, Harvey RP, Moorman AFM, Christoffels VM (2007) Molecular pathway for the localized formation of the sinoatrial node. Circ Res 100:354–362. https://doi.org/10.1161/01.RES.0000258019.74591.b3
Nakayama T, Kurachi Y, Noma A, Irisawa H (1984) Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch - Eur J Physiol 402:248–257. https://doi.org/10.1007/BF00585507
Needham J (1970) Developmental landmarks in cardiac morphogenesis: comparative chronology. Am J Cardiol 25:141–148
Occhetta E, Dell’Era G, Degiovanni A, Sartori C (2015) Persistence of left superior vena cava and focal right atrial tachycardia: challenges and interventional treatment. Cor Vasa 57:e354–e358. https://doi.org/10.1016/j.crvasa.2015.05.008
Olsen KB, Axelsen LN, Braunstein TH, Sørensen CM, Andersen CB, Ploug T, Holstein-Rathlou NH, Nielsen MS (2013) Myocardial impulse propagation is impaired in right ventricular tissue of Zucker Diabetic Fatty (ZDF) rats. Cardiovasc Diabetol 12:1–11. https://doi.org/10.1186/1475-2840-12-19
Oosthoek PW, Viragh S, Mayen AEM, van Kempen MJA, Lamers WH, We AFMM (1993) Immunohistochemical delineation of the conduction system: I: the sinoatrial node. Circ Res 73:473–481. https://doi.org/10.1161/01.RES.73.3.473
Piffer CR, Piffer MIS, Santi FP, Dayoub MCO (1996) Structural Characteristics of the superior intrapericardium segment in adults venae cavae wall at the and aging individuals. Okajimas Folia Anat 73:89–100
Quigley KS, Shair HN, Myers MM (1996) Parasympathetic control of heart period during early postnatal development in the rat. J Auton Nerv Syst 59(1–2):75–82. https://doi.org/10.1016/0165-1838(96)00010-0
Rohr S, Kucera JP, Kléber AG, Rohr S, Kucera JP, Kle G (1998) Slow conduction in cardiac tissue, I: Effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res 83:781–794. https://doi.org/10.1161/01.RES.83.8.781
Sharma SP, Sangha RS, Dahal K, Krishnamoorthy P (2017) The role of empiric superior vena cava isolation in atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol 48:61–67. https://doi.org/10.1007/s10840-016-0198-2
Shaw RM, Rudy Y (1997) Ionic mechanisms of propagation in cardiac tissue. Circ Res 81:727–741. https://doi.org/10.1161/01.RES.81.5.727
Shinagawa Y, Satoh H, Noma A (2000) The sustained inward current and inward rectifier K+ current in pacemaker cells dissociated from rat sinoatrial node. J Physiol 523:593–605
Weingart R, Bukauskas FF (1998) Long-chain n-alkanols and arachidonic acid interfere with the V(m)-sensitive gating mechanism of gap junction channels. Pflugers Arch - Eur J Physiol 435:310–319. https://doi.org/10.1007/s004240050517
Yeh HI, Lai YJ, Lee SH, Lee YN, Ko YS, Chen SA, Severs NJ, Tsai CH (2001) Heterogeneity of myocardial sleeve morphology and gap junctions in canine superior vena cava. Circulation 104:3152–3157. https://doi.org/10.1161/hc5001.100836
Zehendner CM, Luhmann HJ, Yang JW (2013) A simple and novel method to monitor breathing and heart rate in awake and urethane-anesthetized newborn rodents. PLoS One 8:1–9. https://doi.org/10.1371/journal.pone.0062628
Funding
The study was supported by the Russian Foundation for Basic Research (RFBR) (grant no. 18-315-00253).
Author information
Authors and Affiliations
Contributions
Alexandra D. Ivanova carried out electrophysiological experiments, data analysis, and drafted the manuscript. Daria V. Samoilova performed the immunohistochemical staining. Artem A. Razumov carried out the analysis of optical mapping data. Vlad S. Kuzmin conceived and designed the study and drafted and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All experimental procedures were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and approved by the Ethics Committee of the Biological faculty of MSU.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivanova, A.D., Samoilova, D.V., Razumov, A.A. et al. Rat caval vein myocardium undergoes changes in conduction characteristics during postnatal ontogenesis. Pflugers Arch - Eur J Physiol 471, 1493–1503 (2019). https://doi.org/10.1007/s00424-019-02320-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02320-0