Abstract
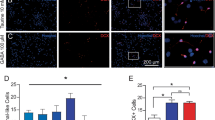
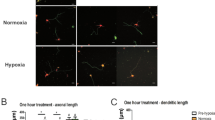
Oligodendrocytes, which differentiate from oligodendrocyte precursor cells (OPCs), ensheath axons with myelin, play an essential role in rapid conduction of action potentials and metabolically support neurons. Elucidation of the mechanisms underlying the proliferation, migration, differentiation, and survival of OPCs is considered indispensable for determining the causes of central nervous system diseases. However, the relationship between these functions of OPCs and their intracellular Ca2+ signaling has not been fully elucidated. Here, we investigated the function of transient receptor potential vanilloid 4 (TRPV4), a Ca2+-permeable channel that responds to hypo-osmolarity, mild temperature, mechanical stimulation, and endogenous arachidonic acid metabolites, in OPCs. Trpv4 mRNA was detected in OPCs in vivo and in primary cultured rat OPCs. In Ca2+ imaging experiments, treatment with the selective TRPV4 agonist GSK1016790A induced sustained elevation of the intracellular Ca2+ concentration in OPCs in a concentration-dependent manner, which was almost completely suppressed by co-treatment with the selective TRPV4 antagonist HC067047. Stimulation of TRPV4 by GSK1016790A augmented OPC proliferation, which was abolished by co-treatment with HC067047, the intracellular Ca2+ chelator BAPTA-AM, and the protein kinase C inhibitor bisindolylmaleimide II. By contrast, GSK1016790A did not significantly affect the migration or differentiation of OPCs. Taken together, these results suggest that TRPV4 is functionally expressed in OPCs and increases the proliferation of these cells without affecting their ability to differentiate into oligodendrocytes.





Similar content being viewed by others
References
Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD (2008) Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 28:1046–1057. https://doi.org/10.1523/JNEUROSCI.4497-07.2008
Alpizar YA, Boonen B, Sanchez A, Jung C, López-Requena A, Naert R, Steelant B, Luyts K, Plata C, De Vooght V, Vanoirbeek JAJ, Meseguer VM, Voets T, Alvarez JL, Hellings PW, Hoet PHM, Nemery B, Valverde MA, Talavera K (2017) TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat Commun 8:1059. https://doi.org/10.1038/s41467-017-01201-3
Baron W, Metz B, Bansal R, Hoekstra D, de Vries H (2000) PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci 15:314–329. https://doi.org/10.1006/mcne.1999.0827
Bhat NR, Hauser KF, Kindy MS (1992) Cell proliferation and protooncogene induction in oligodendroglial progenitors. J Neurosci Res 32:340–349. https://doi.org/10.1002/jnr.490320306
Chen LX, Ma SM, Zhang P, Fan ZC, Xiong M, Cheng GQ, Yang Y, Qiu ZL, Zhou WH, Li J (2015) Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PLoS One 10:e0115997. https://doi.org/10.1371/journal.pone.0115997
Cheli VT, Santiago González DA, Spreuer V, Paez PM (2015) Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp Neurol 265:69–83. https://doi.org/10.1016/j.expneurol.2014.12.012
Cheli VT, Santiago González DA, Namgyal Lama T, Spreuer V, Handley V, Murphy GG, Paez PM (2016) Conditional deletion of the L-type calcium channel Cav1.2 in oligodendrocyte progenitor cells affects postnatal myelination in mice. J Neurosci 36:10853–10869. https://doi.org/10.1523/JNEUROSCI.1770-16.2016
Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T (2003) Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol 163:397–408. https://doi.org/10.1083/jcb.200304154
Cui QL, Kuhlmann T, Miron VE, Leong SY, Fang J, Gris P, Kennedy TE, Almazan G, Antel J (2013) Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am J Pathol 183:516–525. https://doi.org/10.1016/j.ajpath.2013.04.016
Fannon J, Tarmier W, Fulton D (2015) Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63:1021–1035. https://doi.org/10.1002/glia.22799
Faulkner J, Keirstead HS (2005) Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol 15:131–142. https://doi.org/10.1016/j.trim.2005.09.007
Fekete Z, Csernai M, Kocsis K, Horváth ÁC, Pongrácz A, Barthó P (2017) Simultaneous in vivo recording of local brain temperature and electrophysiological signals with a novel neural probe. J Neural Eng 14:034001. https://doi.org/10.1088/1741-2552/aa60b1
Ferent J, Zimmer C, Durbec P, Ruat M, Traiffort E (2013) Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J Neurosci 33:1759–1772. https://doi.org/10.1523/JNEUROSCI.3334-12.2013
Fornari E, Maeder P, Meuli R, Ghika J, Knyazeva MG (2012) Demyelination of superficial white matter in early Alzheimer’s disease: a magnetization transfer imaging study. Neurobiol Aging 428:e7–19. https://doi.org/10.1016/j.neurobiolaging.2010.11.014
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354:942–955. https://doi.org/10.1056/NEJMra052130
Hamilton N, Vayro S, Wigley R, Butt AM (2010) Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 58:66–79. https://doi.org/10.1002/glia.20902
Hamilton NB, Kolodziejczyk K, Kougioumtzidou E, Attwell D (2016) Proton-gated Ca(2+)-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 529:523–527. https://doi.org/10.1038/nature16519
Hoffmann A, Grimm C, Kraft R, Goldbaum O, Wrede A, Nolte C, Hanisch UK, Richter-Landsberg C, Brück W, Kettenmann H, Harteneck C (2010) TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J Neurochem 114:654–665. https://doi.org/10.1111/j.1471-4159.2010.06644.x
Jahan MGS, Yumura S (2017) Traction force and its regulation during cytokinesis in Dictyostelium cells. Eur J Cell Biol 96:515–528. https://doi.org/10.1016/j.ejcb.2017.06.004
Kiyatkin EA, Brown PL, Wise RA (2002) Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci 16:164–168
Konno M, Shirakawa H, Iida S, Sakimoto S, Matsutani I, Miyake T, Kageyama K, Nakagawa T, Shibasaki K, Kaneko S (2012) Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia 60:761–770. https://doi.org/10.1002/glia.22306
Kuroda M, Muramatsu R, Maedera N, Koyama Y, Hamaguchi M, Fujimura H, Yoshida M, Konishi M, Itoh N, Mochizuki H, Yamashita T (2017) Peripherally derived FGF21 promotes remyelination in the central nervous system. J Clin Invest 127:3496–3509. https://doi.org/10.1172/JCI94337
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. https://doi.org/10.1038/nature11314
Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C (2013) A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61:732–749. https://doi.org/10.1002/glia.22469
Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Ffrench-Constant C, Attwell D, Káradóttir RT (2013) Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol 11:e1001743. https://doi.org/10.1371/journal.pbio.1001743
Ma J, Matsumoto M, Tanaka KF, Takebayashi H, Ikenaka K (2006) An animal model for late onset chronic demyelination disease caused by failed terminal differentiation of oligodendrocytes. Neuron Glia Biol 2:81–91. https://doi.org/10.1017/S1740925X06000056
Ma J, Tanaka KF, Yamada G, Ikenaka K (2007) Induced expression of cathepsins and cystatin C in a murine model of demyelination. Neurochem Res 32:311–320. https://doi.org/10.1007/s11064-006-9183-y
McTigue DM, Wei P, Stokes BT (2001) Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci 21:3392–3400
Miyake T, Nakamura S, Zhao M, So K, Inoue K, Numata T, Takahashi N, Shirakawa H, Mori Y, Nakagawa T, Kaneko S (2016) Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat Commun 7:12840. https://doi.org/10.1038/ncomms12840
Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M (2009) The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 287:21257–21264. https://doi.org/10.1074/jbc.M109.020206
Najjar S, Pearlman DM (2016) Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161:102–112. https://doi.org/10.1016/j.schres.2014.04.041
Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B (2013) Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol 15:602–613. https://doi.org/10.1038/ncb2736
Paez PM, Fulton DJ, Spreur V, Handley V, Campagnoni AT (2010) Multiple kinase pathways regulate voltage-dependent Ca2+ influx and migration in oligodendrocyte precursor cells. J Neurosci 30:6422–6433. https://doi.org/10.1523/JNEUROSCI.5086-09.2010
Paez PM, Fulton D, Spreuer V, Handley V, Campagnoni AT (2011) Modulation of canonical transient receptor potential channel 1 in the proliferation of oligodendrocyte precursor cells by the golli products of the myelin basic protein gene. J Neurosci 31:3625–3637. https://doi.org/10.1523/JNEUROSCI.4424-10.2011
Paez PM, Cheli VT, Ghiani CA, Spreuer V, Handley VW, Campagnoni AT (2012) Golli myelin basic proteins stimulate oligodendrocyte progenitor cell proliferation and differentiation in remyelinating adult mouse brain. Glia 60:1078–1093. https://doi.org/10.1002/glia.22336
Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, Tombola F (2014) Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A 111:16148–16153. https://doi.org/10.1073/pnas.1409802111
Radhakrishna M, Almazan G (1994) Protein kinases mediate basic fibroblast growth factor’s stimulation of proliferation and c-fos induction in oligodendrocyte progenitors. Brain Res Mol Brain Res 24:118–128
Shibasaki K, Suzuki M, Mizuno A, Tominaga M (2007) Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 27:1566–1575. https://doi.org/10.1523/JNEUROSCI.4284-06.2007
Shibasaki K, Takebayashi H, Ikenaka K, Feng L, Gan L (2007) Expression of the basic helix-loop-factor Olig2 in the developing retina: Olig2 as a new marker for retinal progenitors and late-born cells. Gene Expr Patterns 7:57–65. https://doi.org/10.1016/j.modgep.2006.05.008
Shibasaki K, Ikenaka K, Tamalu F, Tominaga M, Ishizaki Y (2014) A novel subtype of astrocytes expressing TRPV4 (transient receptor potential vanilloid 4) regulates neuronal excitability via release of gliotransmitters. J Biol Chem 289:14470–14480. https://doi.org/10.1074/jbc.M114.557132
Shibasaki K (2016) TRPV4 ion channel as important cell sensors. J Anesth 30:1014–1019. https://doi.org/10.1007/s00540-016-2225-y
Shirakawa H, Katsumoto R, Iida S, Miyake T, Higuchi T, Nagashima T, Nagayasu K, Nakagawa T, Kaneko S (2017) Sphingosine-1-phosphate induces Ca2+ signaling and CXCL1 release via TRPC6 channel in astrocytes. Glia 65:1005–1016. https://doi.org/10.1002/glia.23141
Sypecka J, Sarnowska A (2014) The neuroprotective effect exerted by oligodendroglial progenitors on ischemically impaired hippocampal cells. Mol Neurobiol 49:685–701. https://doi.org/10.1007/s12035-013-8549-9
Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424:434–438. https://doi.org/10.1038/nature01807
Waxman SG, Bennett MV (1972) Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat New Biol 238(85):217–219
Wu LJ, Sweet TB, Clapham DE (2010) International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62:381–404. https://doi.org/10.1124/pr.110.002725
Zheng F, Xia ZA, Zeng YF, Luo JK, Sun P, Cui HJ, Wang Y, Tang T, Zhou YT (2017) Plasma metabolomics profiles in rats with acute traumatic brain injury. PLoS One 12:e0182025. https://doi.org/10.1371/journal.pone.0182025
Acknowledgments
We appreciate Dr. H. Takebayashi (Niigata Univ.) for the generous gift of PDGFRα, plp, and gfap ISH plasmids, and Dr. K. Arai (Harvard Univ.) for his excellent advice on primary culture of oligodendrocyte precursor cells.
Funding
This work was supported by MEXT/JSPS KAKENHI Grant Numbers 17K19486 (to H.S.), 24390016 (to S.K.), JP15H05934 <Thermal Biology> (to K.S.), and JP15H03000 (to K.S.), and also supported by the Takeda Science Foundation and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to H.S.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted in accordance with the ethical guidelines set down by the Kyoto University Animal Research Committee.
Additional information
This article is part of the special issue on Thermal biology in Pflügers Archiv – European Journal of Physiology
Rights and permissions
About this article
Cite this article
Ohashi, K., Deyashiki, A., Miyake, T. et al. TRPV4 is functionally expressed in oligodendrocyte precursor cells and increases their proliferation. Pflugers Arch - Eur J Physiol 470, 705–716 (2018). https://doi.org/10.1007/s00424-018-2130-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2130-3