Abstract
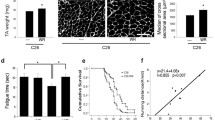
Cisplatin, a platinum-based anti-cancer drug, is one of the most effective broad-spectrum anti-cancer agents used against various cancers. It has been recently suggested that low skeletal muscle mass is predictive of mortality in patients with cancer. Although several molecules produced by the actual tumor itself contribute to skeletal muscle impairment, we recently suggested that the administration of cisplatin could increase levels of muscle RING finger-1 (MuRF1) and atrogin-1, possibly leading to muscle atrophy in the mouse. Exercise is an important factor that induces muscle protein synthesis and muscle hypertrophy by enhancing the positive effects of the Akt/mTOR/p70S6 kinase pathway. In the present study, we therefore investigated the effect of treadmill exercise on cisplatin-induced muscle atrophy. C57BL/6J mice were treated with cisplatin (3 mg/kg, i.p.) or saline for four consecutive days. On day 4, the quadriceps and gastrocnemius muscles were isolated from the mice. The animals in the treadmill exercise groups were forced to run on a motorized treadmill for 20 min once a day for 9 days. In addition to muscle mass, the decrease in myofiber diameter associated with cisplatin administration was significantly restored by treadmill exercise. This exercise also significantly attenuated cisplatin-induced upregulation of MuRF1 and atrogin-1 in quadriceps and gastrocnemius muscle. The decreased Akt, p70S6 kinase, and Foxo3a phosphorylation observed with cisplatin treatment was significantly recovered by treadmill exercise in both the muscles. In the present study, myostatin (Mstn) gene expression, upregulated by cisplatin administration, was also attenuated by treadmill exercise. These findings suggest that treadmill exercise could attenuate cisplatin-induced muscle atrophy, at least partially, and could improve prognosis.







Similar content being viewed by others
References
Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, Radlgruber I, Mayerhoefer ME, Grimm C, Polterauer S (2015) Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS One 10:e0140403
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6:25–39
Costelli P, Muscaritoli M, Bossola M, Moore-Carrasco R, Crepaldi S, Grieco G, Autelli R, Bonelli G, Pacelli F, Lopez-Soriano FJ, Argiles JM, Doglietto GB, Baccino FM, Rossi Fanelli F (2005) Skeletal muscle wasting in tumor-bearing rats is associated with MyoD down-regulation. Int J Oncol 26:1663–1668
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576:613–624
Foletta VC, White LJ, Larsen AE, Leger B, Russell AP (2011) The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch 461:325–335
Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–140
Glass DJ (2010) PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol 346:267–278
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98:14440–14445
Jee H, Chang JE, Yang EJ (2016) Positive prehabilitative effect of intense treadmill exercise for ameliorating cancer cachexia symptoms in a mouse model. J Cancer 7:2378–2387
Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O (2002) Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol (1985) 93:1301–1309
Kim JS, Cross JM, Bamman MM (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288:E1110–E1119
Lecker SH, Goldberg AL, Mitch WE (2006) Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17:1807–1819
Lecker SH, Solomon V, Mitch WE, Goldberg AL (1999) Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129:227S–237S
Manttari S, Jarvilehto M (2005) Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC Physiol 5:4
Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E (2008) Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab 294:E43–E51
McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514
Minetti GC, Feige JN, Rosenstiel A, Bombard F, Meier V, Werner A, Bassilana F, Sailer AW, Kahle P, Lambert C, Glass DJ, Fornaro M (2011) Galphai2 signaling promotes skeletal muscle hypertrophy, myoblast differentiation, and muscle regeneration. Sci Signal 4:ra80
Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M (2005) Atrophy of S6K1(-/-) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7:286–294
Okita M, Yoshimura T, Nakano J, Watabe M, Nagai T, Kato K, Eguchi K (2001) Effects of treadmill exercise on muscle fibers in mice with steroid myopathy. J Jpn Phys Ther Assoc 4:25–27
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, Baracos VE (2013) Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 98:1012–1019
Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, Seiliez I, Picard B, Bonnieu A (2014) Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci 71:4361–4371
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013
Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA (2003) Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 228:706–709
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151:1319–1331
Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S (2011) Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 18:3579–3585
Sakai H, Sagara A, Arakawa K, Sugiyama R, Hirosaki A, Takase K, Jo A, Sato K, Chiba Y, Yamazaki M, Matoba M, Narita M (2014) Mechanisms of cisplatin-induced muscle atrophy. Toxicol Appl Pharmacol 278:190–199
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531
Senf SM, Dodd SL, Judge AR (2010) FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 298:C38–C45
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Suzuki N, Motohashi N, Uezumi A, Fukada S, Yoshimura T, Itoyama Y, Aoki M, Miyagoe-Suzuki Y, Takeda S (2007) NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest 117:2468–2476
Tisdale MJ (2010) Cancer cachexia. Curr Opin Gastroenterol 26:146–151
Welle S, Burgess K, Mehta S (2009) Stimulation of skeletal muscle myofibrillar protein synthesis, p70 S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation by inhibition of myostatin in mature mice. Am J Physiol Endocrinol Metab 296:E567–E572
Acknowledgments
This work was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411019) and by KAKENHI (15K18880), a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
HS, MK, YI, SY, AM, YT, and YK performed the experiments and analyzed the data. FS, TY, YC, and MN provided technical support. HS and MN initiated and supervised the study. HS and MN designed experiments and analyzed data. HS and YC wrote the manuscript.
Corresponding author
Ethics declarations
All experiments were approved by the Animal Care Committee at Hoshi University (Tokyo, Japan).
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sakai, H., Kimura, M., Isa, Y. et al. Effect of acute treadmill exercise on cisplatin-induced muscle atrophy in the mouse. Pflugers Arch - Eur J Physiol 469, 1495–1505 (2017). https://doi.org/10.1007/s00424-017-2045-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2045-4