Abstract
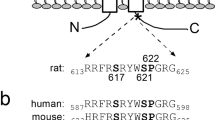
The serum- and glucocorticoid-inducible kinase 1 (SGK1) has been identified as a signalling molecule up-regulated by aldosterone, which stimulates the renal epithelial Na+ channel ENaC. It is therefore thought to participate in the antinatriuretic action of this hormone. More recently, two isoforms, SGK2 and SGK3, have been cloned. The present study was performed to establish whether SGK2 and SGK3 influence ENaC activity similarly to SGK1. Dual-electrode voltage-clamp experiments in Xenopus laevis oocytes expressing α,ß,γ-ENaC with or without SGK1, SGK2 or SGK3 revealed a stimulatory effect of all three kinases on the amiloride-sensitive current (I Na). To establish whether the SGK isoforms exert their effects through direct phosphorylation, we replaced the serine at the SGK consensus site of αENaC (αS622AENaC) by site-directed mutagenesis. αS622A,β,γ-ENaC was up-regulated similar to wild-type ENaC, suggesting that SGK isoforms do not act via direct phosphorylation of the transport proteins. In conclusion, SGK2 and SGK3 mimic the function of SGK1 and are likely to participate in the regulation of ENaC activity.



Similar content being viewed by others
References
Blazer-Yost BL, Cox M (1988) Insulin-like growth factor 1 stimulates renal epithelial Na+ transport. Am J Physiol 255:C413–C417
Blazer-Yost BL, Shah N, Jarred L, Cox M, Smith RM (1972) Insulin and IGF1 receptors in a model renal epithelium: receptor localization and characterization. Biochem Int 28:143–153
Blazer-Yost BL, Record RD, Oberleithner H (1996) Characterization of hormone stimulated Na+ transport in a high-resistance clone of the MDCK cell line. Pflugers Arch 432:685–691
Böhmer C, Wagner CA, Beck S, Moschen I, Melzig J, Werner A, Lin JT, Lang F, Wehner F (2000) The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol Biochem 10:187–194
Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D (1999) Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96:2514–2519
De la Rosa DA, Zhang P, Naray-Fejes-Toth A, Fejes-Toth G, Canessa CM (1999) The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J Biol Chem 274:37834–37839
Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O (2001) Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20:7052–7059
Kobayashi T, Cohen P (1999) Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339:319–328
Kobayashi T, Deak M, Morrice N, Cohen P (1999) Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344:189–197
Lang F, Klingel K, Wagner CA, Stegen C, Wärntges S, Friedrich B, Lanzendörfer M, Melzig J, Moschen I, Steuer S, Waldegger S, Sauter M, Paulmichl M, Gerke V, Risler T, Gamba G, Capasso G, Kandolf R, Hebert SC, Massry SG, Bröer S (2000) Deranged transcriptional regulation of cell volume sensitive kinase hSGK in diabetic nephropathy. Proc Natl Acad Sci USA 97:8157–8162
Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G (1999) sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem 274:16973–16978
Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA (1999) Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18:3024–3033
Shigaev A, Asher C, Latter H, Garty H, Reuveny E (2000) Regulation of sgk by aldosterone and its effects on the epithelial Na+ channel. Am J Physiol 278:F613–F619
Wagner CA, Friedrich B, Setiawan I, Lang F, Bröer S (2000) The use of Xenopus laevis oocytes for the functional characterization of heterologously expressed membrane proteins. Cell Physiol Biochem 10:1–12
Waldegger S, Barth P, Raber G, Lang F (1997) Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA 94:4440–4445
Waldegger S, Barth P, Forrest JN Jr, Greger R, Lang F (1998) Cloning of sgk serine-threonine protein kinase from shark rectal gland a gene induced by hypertonicity and secretagogues. Pflugers Arch 436:575–580
Waldegger S, Gabrysch S, Barth P, Fillon S, Lang F (2000) h-sgk serine-threonine protein kinase as transcriptional target of p38/MAP kinase pathway in HepG2 human hepatoma cells. Cell Physiol Biochem 10:203–208
Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL (1993) Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13:2031–2040
Acknowledgements
The authors are indebted to Dr. B. Rossier (Lausanne, Switzerland) for providing the ENaC constructs. They acknowledge the technical assistance of B. Noll and the meticulous preparation of the manuscript by Leijla Subasic. This study was supported by the Deutsche Forschungsgemeinschaft, Nr. La 315/4-4 and La 315/5-1, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Centre for Interdisciplinary Clinical Research) 01 KS 9602 (to FL) and the UK Medical Research Council and The Royal Society (to PC).
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Friedrich and Y. Feng share first authorship
Rights and permissions
About this article
Cite this article
Friedrich, B., Feng, Y., Cohen, P. et al. The serine/threonine kinases SGK2 and SGK3 are potent stimulators of the epithelial Na+ channel α,β,γ-ENaC. Pflugers Arch - Eur J Physiol 445, 693–696 (2003). https://doi.org/10.1007/s00424-002-0993-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-0993-8