Abstract
Purpose
Research projects and clinical trials strongly rely on high-quality biospecimens which are provided by biobanks. Since differences in sample processing and storage can strongly affect the outcome of such studies, standardization between biobanks is necessary to guarantee reliable results of large, multicenter studies. The German Cancer Aid Foundation (Deutsche Krebshilfe e.V.) has therefore initiated the priority program “tumor tissue banks” in 2010 by funding four biobank networks focusing on central nervous system tumors, melanomas, breast carcinomas, and colorectal carcinomas. The latter one, the North German Tumor Bank of Colorectal Cancer (ColoNet) is managed by surgeons, pathologists, gastroenterologists, oncologists, scientists, and medical computer scientists.
Methods and results
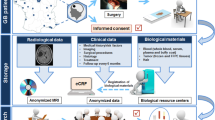
The ColoNet consortium has developed and harmonized standard operating procedures concerning all biobanking aspects. Crucial steps for quality assurance have been implemented and resulted in certification according to DIN EN ISO 9001. A further achievement is the construction of a web-based database for exploring available samples. In addition, common scientific projects have been initiated. Thus, ColoNet’s repository will be used for research projects in order to improve early diagnosis, therapy, follow-up, and prognosis of colorectal cancer patients. Apart from the routine sample storage at −170 °C, the tumor banks’ unique characteristic is the participation of outpatient clinics and private practices to further expand the sample and clinical data collection.
Conclusion
The first 2 years of funding by the German Cancer Aid Foundation have already led to a closer scientific connection between the participating institutions and to a substantial collection of biospecimens obtained under highly standardized conditions.




Similar content being viewed by others
References
Sotiriou C, Piccart MJ (2007) Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer 7(7):545–553. doi:10.1038/nrc2173
Marko-Varga G (2011) Biobanking—the holy grail of novel drug and diagnostic developments? J Clin Bioinforma 1(1):14. doi:10.1186/2043-9113-1-14
Hewitt R, Hainaut P (2011) Biobanking in a fast moving world: an international perspective. J Natl Cancer Inst Monogr 2011(42):50–51. doi:10.1093/jncimonographs/lgr005
Hewitt RE (2011) Biobanking: the foundation of personalized medicine. Curr Opin Oncol 23(1):112–119. doi:10.1097/CCO.0b013e32834161b8
Gaffney EF, Madden D, Thomas GA (2012) The human side of cancer biobanking. Methods Mol Biol 823:59–77. doi:10.1007/978-1-60327-216-2_5
Stege A, Hummel M (2008) Experience with establishment and operation of a biobank. Der Pathologe 29(Suppl 2):214–217. doi:10.1007/s00292-008-1043-x
Bevilacqua G, Bosman F, Dassesse T, Hofler H, Janin A, Langer R, Larsimont D, Morente MM, Riegman P, Schirmacher P, Stanta G, Zatloukal K, Caboux E, Hainaut P (2010) The role of the pathologist in tissue banking: European Consensus Expert Group Report. Virchows Arch Int J Pathol 456(4):449–454. doi:10.1007/s00428-010-0887-7
Asslaber M, Zatloukal K (2007) Biobanks: transnational, European and global networks. Brief Funct Genomic Proteomic 6(3):193–201. doi:10.1093/bfgp/elm023
Betsou F, Lehmann S, Ashton G, Barnes M, Benson EE, Coppola D, DeSouza Y, Eliason J, Glazer B, Guadagni F, Harding K, Horsfall DJ, Kleeberger C, Nanni U, Prasad A, Shea K, Skubitz A, Somiari S, Gunter E (2010) Standard preanalytical coding for biospecimens: defining the sample PREanalytical code. Cancer Epidemiol Biomarkers Prev: A Publ Am Assoc Cancer Res, Cosponsored by the Am Soc Prev Oncol 19(4):1004–1011. doi:10.1158/1055-9965.EPI-09-1268
Vegvari A, Welinder C, Lindberg H, Fehniger TE, Marko-Varga G (2011) Biobank resources for future patient care: developments, principles and concepts. J Clin Bioinforma 1(1):24. doi:10.1186/2043-9113-1-24
Riegman PH, Morente MM, Betsou F, de Blasio P, Geary P (2008) Biobanking for better healthcare. Mol Oncol 2(3):213–222. doi:10.1016/j.molonc.2008.07.004
Schmitt M, Mengele K, Schueren E, Sweep FC, Foekens JA, Brunner N, Laabs J, Malik A, Harbeck N (2007) European Organisation for Research and Treatment of Cancer (EORTC) Pathobiology Group standard operating procedure for the preparation of human tumour tissue extracts suited for the quantitative analysis of tissue-associated biomarkers. Eur J Cancer 43(5):835–844. doi:10.1016/j.ejca.2007.01.008
Linnebacher M, Maletzki C, Ostwald C, Klier U, Krohn M, Klar E, Prall F (2010) Cryopreservation of human colorectal carcinomas prior to xenografting. BMC Cancer 10:362. doi:10.1186/1471-2407-10-362
Betsou F, Luzergues A, Carter A, Geary P, Riegman P, Clark B, Morente M, Vaught J, Dhirr R, Druez-Vérité C (2007) Towards norms for accreditation of biobanks for human health and medical research: compilation of existing guidelines into an ISO certification/accreditation norm-compatible format. Qual Assur J 11(3–4):221–294. doi:10.1002/qaj.425
Carter A, Betsou F, Clark BJ (2011) Quality management and accreditation of research tissue banks. Virchows Arch Int J Pathol 458(2):247–248. doi:10.1007/s00428-010-1028-z, author reply 249–250
Gerling M, Meyer KF, Fuchs K, Igl BW, Fritzsche B, Ziegler A, Bader F, Kujath P, Schimmelpenning H, Bruch HP, Roblick UJ, Habermann JK (2010) High frequency of aneuploidy defines ulcerative colitis-associated carcinomas: a comparative prognostic study to sporadic colorectal carcinomas. Ann Surg. doi:10.1097/SLA.0b013e3181deb664
Pantel K, Alix-Panabieres C (2010) Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 16(9):398–406. doi:10.1016/j.molmed.2010.07.001
Vegas H, Andre T, Bidard FC, Ferrand FR, Huguet F, Mariani P, Pierga JY (2012) Disseminated and circulating tumor cells in gastrointestinal oncology. Bull Cancer 99(5):535–544. doi:10.1684/bdc.2012.1581
Reng CM, Debold P, Specker C, Pommerening K (2006) Generische Lösungen zum Datenschutz für die Forschungsnetze in der Medizin. TMF Schriftenreihe. Medizinisch Wissenschaftliche Verlagsgesellschaft
Murphy S, Churchill S, Bry L, Chueh H, Weiss S, Lazarus R, Zeng Q, Dubey A, Gainer V, Mendis M, Glaser J, Kohane I (2009) Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res 19(9):1675–1681. doi:10.1101/gr.094615.109
Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, Kohane I (2010) Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). J Am Med Inform Assoc: JAMIA 17(2):124–130. doi:10.1136/jamia.2009.000893
Prokosch HU, Ries M, Beyer A, Schwenk M, Seggewies C, Kopcke F, Mate S, Martin M, Barthlein B, Beckmann MW, Sturzl M, Croner R, Wullich B, Ganslandt T, Burkle T (2011) IT infrastructure components to support clinical care and translational research projects in a comprehensive cancer center. Stud Health Technol Inform 169:892–896
Mate S, Burkle T, Kopcke F, Breil B, Wullich B, Dugas M, Prokosch HU, Ganslandt T (2011) Populating the i2b2 database with heterogeneous EMR data: a semantic network approach. Stud Health Technol Inform 169:502–506
Acknowledgments
The tumor biobank consortium “North German Tumor Bank of Colorectal Cancer” is generously supported by the German Cancer Aid Foundation (DKH e.V. #108446).
Conflicts of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Oberländer, M., Linnebacher, M., König, A. et al. The “North German Tumor Bank of Colorectal Cancer”: status report after the first 2 years of support by the German Cancer Aid Foundation. Langenbecks Arch Surg 398, 251–258 (2013). https://doi.org/10.1007/s00423-012-1043-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-1043-4