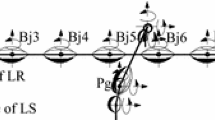
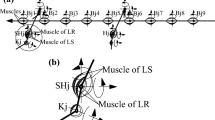
Abstract
Vertebrate animals exhibit impressive locomotor skills. These locomotor skills are due to the complex interactions between the environment, the musculo-skeletal system and the central nervous system, in particular the spinal locomotor circuits. We are interested in decoding these interactions in the salamander, a key animal from an evolutionary point of view. It exhibits both swimming and stepping gaits and is faced with the problem of producing efficient propulsive forces using the same musculo-skeletal system in two environments with significant physical differences in density, viscosity and gravitational load. Yet its nervous system remains comparatively simple. Our approach is based on a combination of neurophysiological experiments, numerical modeling at different levels of abstraction, and robotic validation using an amphibious salamander-like robot. This article reviews the current state of our knowledge on salamander locomotion control, and presents how our approach has allowed us to obtain a first conceptual model of the salamander spinal locomotor networks. The model suggests that the salamander locomotor circuit can be seen as a lamprey-like circuit controlling axial movements of the trunk and tail, extended by specialized oscillatory centers controlling limb movements. The interplay between the two types of circuits determines the mode of locomotion under the influence of sensory feedback and descending drive, with stepping gaits at low drive, and swimming at high drive.




Similar content being viewed by others
Notes
Henceforth the following distinctions are made between locomotor modes (e.g., swimming vs. stepping) and between gaits. The locomotor mode of stepping encompasses (i) at slow speeds, a sequential walking gait, where one foot is lifted off the ground at a time. The order in which the feet are lifted defines the further subdivision in lateral sequence walk (ipsilateral hindlimb follows the ipsilateral forelimb) and diagonal sequence walk (contralateral hindlimb follows the ipsilateral forelimb). (ii) At higher speeds, a walking trot gait, where diagonally opposite feet are lifted roughly simultaneously off the ground. At even higher speeds the walking trot turns into a running trot, with short flight phases (Daan and Belterman 1968; Carrier 1993; Ashley-Ross 1994a).
References
Ashley-Ross MA (1994a) Hindlimb kinematics during terrestrial locomotion in salamander (Dicamptodon tenebrosus). J Exp Biol 193:255–283
Ashley-Ross MA (1994b) Metamorphic and speed effects on hindlimb kinematics during terrestrial locomotion in the salamander Dicamptodon tenebrosus. J Exp Biol 193:285–305
Ashley-Ross MA (1995) Patterns of hind limb motor output during walking in the salamander Dicamptodon tenebrosus, with comparisons to other tetrapods. J Comp Physiol [A] 177:273–285
Ashley-Ross MA, Lauder GV (1997) Motor patterns and kinematics during backward walking in the pacific giant salamander: evidence for novel motor output. J Neurophysiol 78:3047–3060
Ashley-Ross MA, Bechtel BF (2004) Kinematics of the transition between aquatic and terrestrial locomotion in the newt Taricha torosa. J Exp Biol 207:461–474
Ashley-Ross MA, Lundin R, Johnson KL (2009) Kinematics of level terrestrial and underwater walking in the California newt, Taricha torosa. J Exp Zool A Ecol Genet Physiol 311:240–257
Azizi E, Horton JM (2004) Patterns of axial and appendicular movements during aquatic walking in the salamander Siren lacertina. Zoology 107:111–120
Bar-Gad I, Kagan I (1999) Behavior of hindbrain neurons during the transition from rest to evoked locomotion in a newt. Prog Brain Res 123:285–294
Bem T, Cabelguen J-M, Ekeberg Ö, Grillner S (2003) From swimming to walking: a single basic network for two different behaviors. Biol Cybern 88:79–90
Bennett WO, Simons RS, Brainerd EL (2001) Twisting and bending: the functional role of salamander lateral hypaxial musculature during locomotion. J Exp Biol 204:1979–1989
Bicanski A, Ryczko D, Cabelguen JM, Ijspeert AJ (2011) Modeling axial spinal segments of the salamander central pattern generator for locomotion. BMC Neurosci 12(Suppl. 1):P157
Bicanski A, Ryczko D, Cabelguen JM, Ijspeert A (2012) From Lamprey to Salamander: an exploratory modeling study on the architecture of the spinal locomotor networks in the salamander. Biol Cybern. doi:10.1007/s00422-012-0538-y
Bizzi E, Tresch M, Saltiel P (2000) New perspectives on spinal motor systems. Nat Rev Neurosci 1:101–108
Bizzi E, Cheung VCK, d’Avella A, Saltiel P, Tresch M (2008) Combining modules for movement. Brain Res Rev 57:125–133
Bowtell G, Williams T (1991) Anguiliform body dynamics: modelling the interaction between muscle activation and body curvature. Phil Trans R Soc Lond B 334:385–390
Brändle K, Székely G (1973) The control of alternating coordination of limb pairs in the newt (Triturus vulgaris). Brain Behav Evol 8: 366–385
Buchanan JT, Grillner S, Cullheim S, Risling M (1989) Identification of excitatory interneurons contributing to generation of locomotion in lamprey: structure, pharmacology, and function. J Neurophysiol 62:59–69
Buchanan JT (2001) Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog Neurobiol 63: 441–466
Cabelguen J-M, Bourcier-Lucas C, Dubuc R (2003) Bimodal locomotion elicited by electrical stimulation of the midbrain in the salamander Notophthalmus viridescens. J Neurosci 23:2434–2439
Cabelguen J-M, Ijspeert A, Lamarque S, Ryczko D (2010) Axial dynamics during locomotion in vertebrates: lesson from the salamander. Prog Brain Res 187:149–162
Cangiano L, Grillner S (2003) Fast and slow locomotor burst generation in the hemispinal cord of the lamprey. J Neurophysiol 89:2931–2942
Carrier DR (1993) Action of the hypaxial muscles during walking and swimming in the salamander Dicamptodon ensatus. J Exp Biol 180:75–83
Charrier V, Lamarque S, Ryczko D, Cabelguen J-M (2010) Kinematic and electromyographical analysis of the adaptation of locomotor movements in the salamander, Pleurodeles waltlii. 7th Forum of European Neuroscience, Amsterdam
Charrier V, Cabelguen JM (2011) Fictive rhythmic motor patterns generated in tail segments of the adult salamander. Society for Neuroscience, Program 386.04, Abstr. TT15
Chevallier S, Jan Ijspeert A (2008) Organisation of the spinal central pattern generators for locomotion in the salamander: biology and modelling. Brain Res Rev 57:147–161
Chevallier S, Landry M, Nagy F, Cabelguen J-M (2004) Recovery of bimodal locomotion in the spinal-transected salamander, Pleurodeles waltlii. Eur J Neurosci 20:1995–2007
Cheng J, Stein RB, Jovanovic K, Yoshida K, Bennett DJ, Han Y (1998) Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus maculatus) spinal cord. J Neurosci 18:4295–4304
Cheng J, Jovanovic K, Aoyagi Y, Bennett DJ, Han Y, Stein RB (2002) Differential distribution of interneurons in the neural networks that control walking in the mudpuppy (Necturus maculatus) spinal cord. Exp Brain Res 145:190–198
Cheung VC, d’Avella A, Tresch MC, Bizzi E (2005) Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434
Cohen AH, Rossignol S, Grillner S (1988) Neural control of rhythmic movements in vertebrates. Wiley-Interscience
Cohen AH, Ermentrout GB, Kiemel T, Kopell N, Sigvardt KA, Williams TL (1992) Modelling of intersegmental coordination in the lamprey central pattern generator for locomotion. Trends Neurosci 15: 434–438
Currie SN, Stein PS (1990) Cutaneous stimulation evokes long-lasting excitation of spinal interneurons in the turtle. J Neurophysiol 64: 1134–1148
Daan S, Belterman T (1968) Lateral bending in locomotion of some lower tetrapods. Proc Ned Akad Wetten C 71:245–266
D’Août K, Aerts P, DeVree F (1996) The timing of muscle strain and activation during steady swimming in a salamander, Ambystoma mexicanum. Neth J Zool 46:263–271
D’Avella A, Bizzi E (1998) Low dimensionality of supraspinally induced force fields. Proc Natl Acad Sci USA 95:7711–7714
Deban SM, Schilling N (2009) Activity of trunk muscles during aquatic and terrestrial locomotion in Ambystoma maculatum. J Exp Biol 212:2949–2959
Deliagina TG, Zelenin PV, Fagerstedt P, Grillner S, Orlovsky GN (2000) Activity of reticulospinal neurons during locomotion in the freely behaving lamprey. J Neurophysiol 83:853–863
Delvolvé I, Bem T, Cabelguen JM (1997) Epaxial and limb muscle activity during swimming and terrestrial stepping in the adult newt, Pleurodeles waltlii. J Neurophysiol 78:638–650
Delvolvé I, Branchereau P, Dubuc R, Cabelguen JM (1999) Fictive rhythmic motor patterns induced by NMDA in an in vitro brain stem-spinal cord preparation from an adult urodele. J Neurophysiol 82:1074–1077
Dominici N, Ivanenko Y, Cappellini G, d’Avella A (2011) Locomotor primitives in newborn babies and their development. Science 334:997–999
Dubuc R (2009) Locomotor regions in the midbrain (MLR) and diencephalon (DLR). In: Binder MD, Hirokawa N, Windhorst U (eds) Encyclopedia of neuroscience. Springer, Berlin
Dubuc R, Brocard F, Antri M, Fénelon K, Gariépy J-F, Smetana R, Ménard A (2008) Initiation of locomotion in lampreys. Brain Res Rev 57:172–182
Edwards JL (1977) The evolution of terrestrial locomotion. In: Hecht MK, Goody PC, Hecht BM (eds) Major patterns in vertebrate evolution. Plenum Publishing Corp, New York, pp 553–576
Eidelberg E, Walden JG, Nguyen LH (1981) Locomotor control in macaque monkeys. Brain 104:647–663
Ekeberg Ö (1993) A combined neuronal and mechanical model of fish swimming. Biol Cybern 69:363–374
El Manira A, Tegnér J, Grillner S (1994) Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J Neurophysiol 72:1852–1861
Fagerstedt P, Orlovsky GN, Deliagina TG, Grillner S, Ullén F (2001) Lateral turns in the lamprey. II. Activity of reticulospinal neurons during the generation of fictive turns. J Neurophysiol 86:2257–2265
Frolich L, Biewener A (1992) Kinematic and electromyographic analysis of the functional role of the body axis during terrestrial and aquatic locomotion in the salamander Ambystoma tigrinum. J Exp Biol 162:107–130
Gillis G (1997) Anguilliform locomotion in an elongate salamander (Siren intermedia): effects of speed on axial undulatory movements. J Exp Biol 200:767–784
Giszter SF, Mussa-Ivaldi FA, Bizzi E (1993) Convergent force fields organized in the frog’s spinal cord. J Neurosci 13:467–491
Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB (ed) Handbook of physiology, the nervous system. Motor control. Bethesda, Am Physiol Soc, sect. I, vol 2, pp 1179–1236
Grillner S, McClellan A, Sigvardt K (1982) Mechanosensitive neurons in the spinal cord of the lamprey. Brain Res 235:169–173
Grillner S, Williams T, Lagerback PA (1984) The edge cell, a possible intraspinal mechanoreceptor. Science 223:500–503
Grillner S, Wallén P (1985) Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8:linebreak 233–261
Grillner S, Georgopoulos AP, Jordan LM (1997) Selection and initiation of motor behavior. In: Stein PSG, Grillner S, Selverston AI, Stuart DG (eds) Neurons, networks, and motor behavior. MIT Press, Cambridge, pp. 3–19
Grillner S (2006) Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52:751–766
Grillner S (2011) Human locomotor circuits conform. Science 334: 912–913
Guan L, Kiemel T, Cohen AH (2001) Impact of movement and movement-related feedback on the lamprey central pattern generator for locomotion. J Experim Biol 204:2361–2370
Hagevik A, McClellan AD (1994) Coupling of spinal locomotor networks in larval lamprey revealed by receptor blockers for inhibitory amino acids: neurophysiology and computer modeling. J Neurophysiol 72:1810–1829
Harper CE, Roberts A (1993) Spinal cord neuron classes in embryos of the smooth newt Triturus vulgaris: a horseradish peroxidase and immunocytochemical study. Philos Trans R Soc Lond B Biol Sci 340:141–160
Harishandra N, Cabelguen JM, Ekeberg O (2010) A 3D Musculo-mechanical model of the salamander for the study of different gaits and modes of locomotion. Front Neurorobot 4:112
Harischandra N, Knuesel J, Kozlov A, Bicanski A, Cabelguen JM, Ijspeert AJ, Ekeberg Ö (2011) Sensory feedback plays a significant role in generating walking gait and in gait transition in salamanders: a simulation study. Front Neurorobot 5:3
Hill AAV, Masino MA, Calabrese RL (2003) Intersegmental coordination of rhythmic motor patterns. J Neurophysiol 90:531–538
Hubbard CS, Dolence EK, Rose JD (2010) Brainstem reticulospinal neurons are targets for corticotropin-releasing factor-Induced locomotion in roughskin newts. Horm Behav 57:237–246
Ijspeert AJ (2001) A connectionist central pattern generator for the aquatic and terrestrial gaits of a simulated salamander. Biol Cybern 84:331–348
Ijspeert AJ, Crespi A, Cabelguen J-M (2005) Simulation and robotics studies of salamander locomotion: applying neurobiological principles to the control of locomotion in robots. Neuroinf 3:171–195
Ijspeert AJ, Crespi A, Ryczko D, Cabelguen J-M (2007) From swimming to walking with a salamander robot driven by a spinal cord model. Science 315:1416–1420
Ijspeert AJ (2008) Central pattern generators for locomotion control in animals and robots: a review. Neural Netw 21:642–653
Jackson AW, Horinek DF, Boyd MR, McClellan AD (2005) Disruption of left-right reciprocal coupling in the spinal cord of larval lamprey abolishes brain-initiated locomotor activity. J Neurophysiol 94:2031–2044
Jacobson R, Hollyday MA (1982) Electrically evoked walking and fictive locomotion in the chick. J Neurophysiol 48:257–270
Jovanovic K, Burke RE (2004) Morphology of brachial segments in mudpuppy (Necturus maculosus) spinal cord studied with confocal and electron microscopy. J Comp Neurol 471:361–385
Jovanovic K, Petrov T, Stein RB (1999) Effects of inhibitory neurotransmitters on the mudpuppy (Necturus maculatus) locomotor pattern in vitro. Exp Brain Res 129:172–184
Karakasiliotis K, Schilling N, Cabelguen JM, Ijspeert AJ (2012) How far are we in understanding salamander’s locomotion: a kinematics perspective from biology and robotics. Biol Cybern (submitted to—same issue)
Kiemel T, Cohen AH (2001) Bending the lamprey spinal cord causes a slowly-decaying increase in the frequency of fictive swimming. Brain Res 900:57–64
Knuesel J, Ijspeert AJ (2011) Effects of muscle dynamics and proprioceptive feedback on the kinematics and CPG activity of salamander stepping. BMC Neurosci 12(Suppl. 1):P157
Kopell N, Ermentrout GB, Williams TL (1991) On chains of oscillators forced at one end. SIAM J Appl Math 51:1397–1417
Kozlov A, Huss M, Lansner A, Kotaleski JH, Grillner S (2009) Simple cellular and network control principles govern complex patterns of motor behavior. Proc Natl Acad Sci USA 106:20027–20032
Kozlov A, Lansner A, Grillner S, Kotaleski JH (2007) A hemicord locomotor network of excitatory interneurons: a simulation study. Biol Cybern 96(2):229–243
Lamarque S, Ryczko D, Didier H, Cabelguen J-M (2009) Dynamics of the axial locomotor network in intact, freely moving salamanders. Neural Chall. 31st International symposium of GRSNC, Montréal
Lavrov I, Cheng J (2004) Activation of NMDA receptors is required for the initiation and maintenance of walking-like activity in the mudpuppy (Necturus maculatus). Can J Physiol Pharmacol 82:637–644.
Lavrov I, Cheng J (2008) Methodological optimization of applying neuroactive agents for the study of locomotor-like activity in the mudpuppies (Necturus maculatus). J Neurosci Methods 174:97–102
Le Ray D, Juvin L, Ryczko D (2011) Supraspinal control of locomotion: the mesencephalic locomotor region. Prog Brain Res 188:51–70
Lemon RN (2008) Descending pathways in motor control. Annu Rev Neurosci 31:195–218
Loeb G (2001) Learning from the spinal cord. J Physiol 533:111–117
Lowry CA, Rose JD, Moore FL (1996) Corticotropin-releasing factor enhances locomotion and medullary neuronal firing in an amphibian. Horm Behav 30:50–59
Matsushima T, Grillner S (1992) Neural mechanisms of intersegmental coordination in lamprey: local excitability changes modify the phase coupling along the spinal cord. J Neurophysiol 67:373–388
McClellan AD (1986) Command systems for initiating locomotor responses in fish and amphibians—parallels to initiation of locomotion in mammals. In: Grillner S, Stein P, Stuart D, Forssberg H, Herman R (eds) Neurobiology of vertebrate locomotion, Wenner-Gren symposium series. MacMillan Press, London, vol 45, pp. 3–20.
McCrea DA, Rybak IA (2008) Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57:134–146
Muller E, Buessing L, Schemmel J, Meier K (2007) Spike-frequency adapting neural ensembles: beyond mean adaptation and renewal theories. Neural Comput 19:2958–3010
Mullins OJ, Hackett JT, Buchanan JT, Friesen OW (2011) Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model system. Prog Neurobiol 93:244–269
Mussa-Ivaldi FA, Giszter SF, Bizzi E (1994) Linear combinations of primitives in vertebrate motor control. Proc Natl Acad Sci USA 91:7534–7538
Pearson KG (1993) Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16:265–297
Pearson K, Ekeberg Ö, Büschges A (2006) Assessing sensory function in locomotor systems using neuro-mechanical simulations. Trends Neurosci 29:625–631
Peters SE, Goslow GE Jr (1983) From salamanders to mammals: continuity in musculoskeletal function during locomotion. Brain Behav Evol 22:191–197
Rauscent A (2008) Remaniements fonctionnels des réseaux locomoteurs spinaux au cours du développement de l’amphibien Xenopus laevis en métamorphose. PhD thesis, Université Bordeaux 1, Bordeaux
Roos P (1964) Lateral bending in newt locomotion. Proc Ned Akad Wetten C 67:223–232
Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader TA (1998) Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann N Y Acad Sci 860:346–359
Rossignol S, Dubuc R, Gossard JP (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86:89–154
Ryczko D (2008) Dynamique de l’organisation fonctionnelle des réseaux locomoteurs chez la salamandre: du module rythmogène à une palette de modes locomoteurs. PhD thesis, Université de Bordeaux 2, Bordeaux
Ryczko D, Lamarque S, Didier H, Cabelguen JM (2009) Dynamics of the axial locomotor network in the isolated spinal cord of the salamander. Society for Neuroscience, Program 565.8, Abstr. EE6
Ryczko D, Charrier V, Ijspeert A, Cabelguen JM (2010a) Segmental oscillators in axial motor circuits of the salamander: distribution and bursting mechanisms. J Neurophysiol 104:2677–2692
Ryczko D, Dubuc R, Cabelguen J-M (2010b) Rhythmogenesis in axial locomotor networks: an interspecies comparison. Prog Brain Res 187:189–211
Saltiel P, Tresch MC, Bizzi E (1998) Spinal cord modular organization and rhythm generation: an NMDA iontophoretic study in the frog. J Neurophysiol 80:2323–2339
Schroeder DM, Egar MW (1990) Marginal neurons in the urodele spinal cord and the associated denticulate ligaments. J Comp Neurol 301:93–103
Shik ML, Severin FV, Orlovskii GN (1966) Control of walking and running by means of electric stimulation of the midbrain. Biofizika 11:659–666
Skinner RD, Garcia-Rill E (1984) The mesencephalic locomotor region (MLR) in the rat. Brain Res 323:385–389
Steeves JD, Sholomenko GN, Webster DMS (1987) Stimulation of the pontomedullary reticular formation initiates locomotion in decerebrate birds. Brain Res 401:205–212
Székely G, Czéh G (1976) Organisation of Locomotion. In: Llinás RR, Precht W, Capranica RR (eds) Frog neurobiology: a handbook, Springer Verlag, Berlin, pp. 765–792
Székely G, Czéh G, Vörös G (1969) The activity pattern of limb muscles in freely moving normal and deafferented newts. Exp Brain Res 9:53–72
Tresch MC, Bizzi E (1999) Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activiated by low threshold cutaneous stimulation. Exp Brain Res 129:401–416
Tresch MC, Kiehn O (2002) Synchronization of motor neurons during locomotion in the neonatal rat: predictors and mechanisms. J Neurosci 22:9997–10008
Tytell E, Hsu C, Williams T, Cohen AH, Fauci LJ (2010) Interactions between internal forces, body stiffness, and fluid environment in a neuromechanical model of lamprey swimming. Proc Natl Acad Sci USA 107:19832–19837
Uematsu K, Todo T (1997) Identification of the midbrain locomotor nuclei and their descending pathways in the teleost carp. Cyprinus carpio Brain Res 773:1–7
van den Kieboom J (2009) Biped locomotion and stability—a practical approach. Masters thesis, Department of Artificial Intelligence, University of Groningen, the Netherlands
Wallén P, Williams TL (1984) Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J Physiol 347:225–239
Wallén P, Grillner S (1997) Central pattern generators and their interaction with sensory feedback. Proc Am Control Conf 5:2851–2855
Wallén P, Ekeberg O, Lansner A, Brodin L, Tråvén H, Grillner S (1992) A computer-based model for realistic simulations of neural networks. II. The segmental network generating locomotor rhythmicity in the lamprey. J Neurophysiol 68:1939–1950
Wheatley M, Edamura M, Stein RB (1992) A comparison of intact and in vitro locomotion in an adult amphibian. Exp Brain Res 88:609–614
Wheatley M, Jovanovic K, Stein RB, Lawson V (1994) The activity of interneurons during locomotion in the in vitro necturus spinal cord. J Neurophysiol 71:2025–2032
Wheatley M, Stein RB (1992) An in vitro preparation of the mudpuppy for simultaneous intracellular and electromyographic recording during locomotion. J Neurosci Methods 42:129–137
Williams TL, Sigvardt KA, Kopell N, Ermentrout GB, Remler MP (1990) Forcing of coupled nonlinear oscillators: studies of intersegmental coordination in the lamprey locomotor central pattern generator. J Neurophysiol 64:862–871
Acknowledgments
A.B. receives financial supported from the Swiss initiative in systems biology: SystemsX.ch. D. R. receives salary support from the Groupe de Recherche sur le Système Nerveux Central (GRSNC) and the Fonds de la Recherche en Santé du Québec (FRSQ). N.H. acknowledges funding by the Swedish International Development Cooperation Agency. J.K., V.C., Ö.E., A.J.I. and J.-M.C. acknowledge support from the European Community (LAMPETRA Grant: FP7-ICT-2007-1-216100). J.-M.C. further receives support from the Fondation pour la Recherche Médicale (DBC 20101021008). The assistance of H. Didier and S. Lamarque in some experiments is gratefully acknowledged. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article forms part of a special issue of Biological Cybernetics entitled “Lamprey, Salamander Robots and Central Nervous System”.
Rights and permissions
About this article
Cite this article
Bicanski, A., Ryczko, D., Knuesel, J. et al. Decoding the mechanisms of gait generation in salamanders by combining neurobiology, modeling and robotics. Biol Cybern 107, 545–564 (2013). https://doi.org/10.1007/s00422-012-0543-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-012-0543-1