Abstract
The purpose of this study was to investigate associations between acute exercise-induced hormone responses and adaptations to high intensity resistance training in a large cohort (n = 56) of young men. Acute post-exercise serum growth hormone (GH), free testosterone (fT), insulin-like growth factor (IGF-1) and cortisol responses were determined following an acute intense leg resistance exercise routine at the midpoint of a 12-week resistance exercise training study. Acute hormonal responses were correlated with gains in lean body mass (LBM), muscle fibre cross-sectional area (CSA) and leg press strength. There were no significant correlations between the exercise-induced elevations (area under the curve—AUC) of GH, fT and IGF-1 and gains in LBM or leg press strength. Significant correlations were found for cortisol, usually assumed to be a hormone indicative of catabolic drive, AUC with change in LBM (r = 0.29, P < 0.05) and type II fibre CSA (r = 0.35, P < 0.01) as well as GH AUC and gain in fibre area (type I: r = 0.36, P = 0.006; type II: r = 0.28, P = 0.04, but not lean mass). No correlations with strength were observed. We report that the acute exercise-induced systemic hormonal responses of cortisol and GH are weakly correlated with resistance training-induced changes in fibre CSA and LBM (cortisol only), but not with changes in strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein accretion leading to hypertrophy with resistance training is the result of accumulated periods of positive muscle protein balance as a result of the synergistic stimulation of rates of muscle protein synthesis (MPS) by feeding and exercise (Burd et al. 2009; Phillips 2004; Phillips et al. 2009). Nutritional and contractile variables influence changes in MPS and hypertrophy (Phillips 2004; Phillips et al. 2009). For example, the type of protein consumed after resistance exercise can determine the acute amplitude of MPS and lean mass gains (Cribb et al. 2006; Hartman et al. 2007; Tang et al. 2009). Different exercise programs can result in differential responses of MPS after resistance exercise (Burd et al. 2010; Moore et al. 2005). Previously, we reported that milk consumption after resistance exercise performed over 12 weeks resulted in greater increases in muscle fibre cross-sectional area (CSA) and lean body mass (LBM) than consumption of an equivalent amount of soy protein or isoenergetic carbohydrate drink (Hartman et al. 2007). Interestingly, despite the overall group differences we still observed, as is common (Bamman et al. 2007; Petrella et al. 2006, 2008), a high degree of heterogeneity in phenotypic response of gain in LBM, fibre CSA as well as strength. That is, certain individuals ‘responded’ to the resistance training stimulus by exhibiting gains in strength and muscle mass that were sustantially greater than the group mean response despite differences between the groups’ post-exercise nutrition. Thus, in addition to immediate post-exercise nutrition, other factors are clearly contributing to the phenotypic response to resistance training.
Recently, responses of microRNA expression (Davidsen et al. 2010), p70S6K1 phosphorylation (Kumar et al. 2009; Terzis et al. 2008), satellite cell activation and myonuclear addition (Petrella et al. 2008) have been highlighted as exhibiting differentiated responses to resistance training in responders and non-responders. There are also reports (Ahtiainen et al. 2003a; McCall et al. 1999) that the acute growth hormone and testosterone response may also be associated with potential for hypertrophy; however, these studies were based on small sample sizes (n = 7 and 11, respectively) making it difficult to draw definitive conclusions.
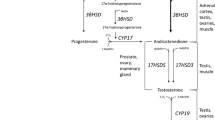
Acute elevations in endogenous hormones (e.g., growth hormone—GH, testosterone, and insulin-like growth factor—IGF-1) are proposed to contribute to resistance training-induced changes in muscle size and strength. Changes in the systemic hormonal milieu are frequently measured since they have been suggested to influence (Beaven et al. 2008a, b; Hansen et al. 2001; Migiano et al. 2009; Ronnestad et al. 2011) and/or predict (Ahtiainen et al. 2003a; McCall et al. 1999) adaptations to resistance exercise. Thus, if the post-exercise hormonal rise influences training adaptations, then individuals who exhibit large hormone responses after resistance exercise would have greater training-induced adaptations and vice versa.
In the present study we aimed, using a large sample size (n = 56), to examine associations between endogenous exercise-induced hormonal responses and resistance exercise training adaptations measured in terms of strength, LBM gain, and muscle fibre hypertrophy. Since our phenotypic outcome data were normally distributed, we propose that this data set would be less prone to the inclusion of outliers and thus more similar to a true population response. We aimed to determine whether the exercise-induced hormone response was associated with the training-induced phenotype (i.e., increase in LBM and muscle fibre CSA), regardless of the nutritional intervention.
Methods
Subjects
A full description of the original methods, study design and subject characteristics, from which the current data are drawn, has been previously published (Hartman et al. 2007). However, we present here the characteristics of our subject pool collapsed across groups since they were analyzed as a single group in terms of responses; see Online Resource 1. Briefly, 56 recreationally active young men, who were not actively participating in any weightlifting activities ≤8 months before the study, were recruited to participate in a 12-week whole-body resistance-training program. Prior to participating, all subjects were informed of the risks associated with the study and gave their written informed consent. The study was approved by the Hamilton Health Sciences Research Ethics Board and conformed to the standards for the use of human subjects in research as outlined in the Declaration of Helsinki as well as to standards established by the Canadian Tri-Council Policy on the ethical use of human subjects (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada, Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, 2010).
Experimental protocol
Full details of the experimental protocol and training program are described elsewhere (Hartman et al. 2007). Briefly, participants trained 5 days per week using several upper- and lower-body exercises. Participants were randomized to one of three post-nutrition groups: skim milk, soy beverage (isonitrogenous, isocaloric, macronutrient matched) or carbohydrate (isocaloric). The drink was consumed immediately and 1 h after each workout as previously described (Hartman et al. 2007). At week 7 of the training program, blood samples were obtained at rest and at 0 (immediately before the first post-exercise supplement), 30, 60, 90 and 120 min after an intense representative bout of lower-body exercise, from a regular training session, for hormone analysis. We have previously shown that there are only minor alterations in the acute hormonal response with resistance training (West et al. 2009) for the hormones we analyzed in the present investigation. Thus, we viewed the characterization of the hormonal response at the midpoint of training as being representative of the training period. All blood draws took place at the same time during the day to prevent diurnal fluctuations in hormones from influencing the responses.
Lean body mass
Changes in LBM were determined as previously described (Hartman et al. 2007) by dual-energy X-ray absorptiometry (QDR-4500A; Hologic Inc, Waltham, MA) scans taken before and after the 12 week training program. Briefly, participants were scanned by the same investigator with the use of bony landmarks and scan table references to ensure consistency of positioning between scans. Coefficients of variation for repeated scans were <2%.
Muscle fibre cross-sectional area
Muscle biopsies were obtained before and after the 12-week training program from the vastus lateralis using a 5-mm Bergström needle that was custom modified for manual suction under local anaesthesia (2% xylocaine). Changes in fibre size were determined by histochemical planimetry as previously described (Hartman et al. 2007; Oates et al. 2010; Shepstone et al. 2005; West et al. 2009). Briefly, muscle fibres were embedded vertically in optimal cutting temperature medium in isopentane cooled with liquid nitrogen. Cross-sections (10 μm thick) were cut and mounted on glass microscope slides for acid pre-incubation (pH = 4.6) and myosin ATPase staining to distinguish type I and II fibres. Stained muscle fibre areas were quantified using ImagePro Plus software (version 4.5.1.22, Media Cybernetics, Bethesda, MD).
Blood analyses
Blood samples were obtained from a catheter inserted into an antecubital vein; the catheter was kept patent by periodic flushes with 0.9% saline. Blood samples were analyzed for serum growth hormone, free testosterone, IGF-1 and cortisol at the Core Laboratory of McMaster University Medical Centre using solid-phase, two site chemiluminescence immunometric assays (Immulite; Intermedico, Holliston, MA). All intra-assay coefficients of variation for these hormones were below 5% and all assays included external and internal standards and daily quality controls. It is important to note that the blood from the original protocol was collected as both serum and plasma and stored continuously at −80°C for the entire time between that completion of the original study and analysis here. We present the hormone concentrations that are uncorrected for changes in plasma volume since these are the concentrations to which potential target tissues are exposed (Judelson et al. 2008; Tremblay et al. 2004).
Statistical analyses
Pearson’s correlation coefficients were generated to determine associative relationships between gains in LBM, fibre CSA and leg press strength, and exercised-induced GH, free testosterone, IGF-1 and cortisol. In light of the dissensus (Curtin and Schulz 1998; Nakagawa 2004) on the statistical thresholds that should be applied to multiple correlations, a single-stage correlation analysis was performed and uncorrected exact P values are presented in order to allow the reader to evaluate the effect size and biological significance of the presented relationships.
In addition to correlation analyses, we also quantified inferential statistics. The original findings of Hartman et al. (2007) showed that gains in LBM were greatest in milk drinkers versus soy or carbohydrate drinkers; however, we aimed to determine whether the responders (i.e., ‘responders’) in each group also had, for example, large acute testosterone or GH responses. Thus, to rule out the possibility that between group differences in hormonal responses may have influenced our findings we calculated standardized Z scores by subtracting the nutritional group (Milk, Soy, Control) mean from the individual data point and dividing the difference by the group standard deviation. Our inclusion criteria for the ‘responder’ category was a Z score that was >+1 and our inclusion criteria for the ‘non-responder’ category was a Z score that was <−1. Hence, if, for example, high testosterone responders were somehow ‘disadvantaged’ by being in the Control group, and testosterone was having an effect on a given training outcome, then by converting to a standardized score based on nutritional group means and standard deviations, we could compare gains in LBM based on testosterone response without the ‘bias’ of the nutritional intervention. In other words, if testosterone were having an effect on LBM gains, irrespective of nutrition, you would expect that those individuals that had higher Z scores would have greater testosterone responses. Thus, we investigated how the hormone response may differ between responders (Z score >+1) and non-responders (Z score <−1) by performing a 1-factor analysis of variance (ANOVA) on the hormone responses based on Z scores of the training adaptation measures.
To gather an overall effect of the hormones on individuals’ training adaptability, we calculated a ‘trainability statistic’ in a similar fashion to the Z score method outlined above, but summed the Z scores of each training outcome (change in LBM, type I and type II fibre area, and leg press 1 repetition maximum strength—1RM) to generate Z sum score. We then compared the hormonal responses of individuals with Z sum > +3 (overall responders) versus those with Z sum < −3 (n = 10 in each group). Similar to the Z score cut-off levels set for the individual training outcomes, Z sum cut-off levels of +3 and −3 were used since they distinguished the same proportion of the sample (i.e., top and bottom ~16%) as ‘responders’ and ‘non-responders’, respectively.
Changes in body weight, fat mass, LBM, and all strength variables, as well as type I and type II fibre area, passed the D’Agostino and Pearson omnibus normality test (all P > 0.05). Paired means (Online Resource 1) were compared using a two-tailed paired Student’s t test. All statistical analyses were performed using SigmaStat 3.1 statistical software (Systat Software Inc, Point Richmond, CA). Values are reported as means ± standard error of the mean (SEM) unless otherwise specified and differences were considered significant at P < 0.05.
Results
Subject physical characteristics, estimated dietary intakes, and single repetition strength pre- and post-training are presented in Online Resource 1. From pre- to post-training, LBM increased 3.1 ± 0.2 kg (P < 0.01; range 0.0–7.6 kg), type I and II fibre area increased 641 ± 60 (range 92–1,845 μm2) and 1123 ± 100 μm2 (39–2,658 μm2), respectively (both P < 0.01), and 1RM strength increased for all exercises (P < 0.001). All references to hormones refer to the exercise-induced hormone response area under the curve (AUC). Consumption of post-exercise nutrition immediately and 60 min after exercise did not appear to have an effect on the exercise-induced hormone response (AUC) (Online Resource 2). Cortisol was positively correlated with change in whole-body LBM (r = 0.29, P < 0.05) whereas GH, free testosterone and IGF-1 were not (Fig. 1). No hormones were associated with change in leg press 1RM strength (Fig. 2). GH was positively correlated with change in type I fibre CSA (r = 0.36, P < 0.01), but cortisol, testosterone and IGF-1 were not (Fig. 3). GH and cortisol were positively correlated with increases in type II fibre area (r = 0.28, P < 0.05; r = 0.35, P < 0.01, respectively), whereas testosterone and IGF-1 were not (Fig. 4).
Analysis of data that was stratified based on standardized Z scores (>+1 = responders and <−1 = non-responders) showed that there were no statistically significant differences in the hormone response between responders and non-responders although there was a trend (P = 0.053) toward a greater IGF-1 response in LBM responders (Fig. 5). Similarly, the hormone responses of overall responders (Z sum > 3) did not differ from the hormone responses of overall non-responders (Z sum < −3; Fig. 5).
Standardized Z scores were calculated using nutritional group (Milk, Soy, Control) means and standard deviations (SD). The GH, free testosterone and IGF-1 response (area under the curve) of individuals with Z scores greater than +1 (i.e., ‘responders’) was compared to the response of individuals with Z scores less than −1 (i.e., ‘non-responders’). A ‘trainability’ statistic was created by summating the Z scores of each training adaptation measure and responders (Z sum > 3) were compared to non-responders (Z sum < −3). There were no differences between responders and non-responders; n = 8–10 for each group
Discussion
We examined associations between the acute exercise-induced elevations of growth hormone, testosterone, IGF-1 and cortisol, measured at the midpoint of a 12 week training program, and training adaptation measures: LBM, muscle fibre CSA, and leg press strength. We found no association between the acute response of any hormone and increase in leg press strength. There was no association between GH or testosterone and the increase in LBM, whereas GH and cortisol were correlated to increases in type II area and explained ~8% and 12% of the variance in this outcome. It should be noted that this data assesses correlations between post-exercise hormone increments and training adaptations, and does not address the possibility of between-subject differences in hormone flux (i.e., secretion, clearance and uptake) and how this relates to individual differences in the propensity to increase strength or hypertrophy with training.
Data points in the correlation analyses were generally dispersed but resulted in positive Pearson correlation coefficients that were the result of net positive ratios of hormone AUC to strength or hypertrophy markers. Given that the correlation is the sum result of all data points, it is possible that there were hormone-adaptation associations in some individuals that were masked by no association in others. The factors that account for individual versus population gains continues to warrant further investigation; the possibility that the combinations of factors underpinning individual adaptation could vary across individuals adds another layer of complexity to our understanding of adaptation to exercise biology.
We extended our correlation analyses by performing an ANOVA on hormone responses that were stratified into standardized scores using the nutritional group means and standard deviations as defined in the original protocol. We found that the hormone responses of individuals who were responders (defined in “Statistical analyses”) for gains in LBM, fibre area and leg press strength were no different from the hormone responses of non-responders. Phrased simply, subjects at the top ~16% in terms of resistance exercise phenotypic responses were no different from those at the bottom ~16% in terms of the acute response of testosterone, GH, IGF-1 and cortisol.
Our hormone analysis is limited to the acute 120 min period after the exercise bout and does not offer insight into potential later (e.g., 24 h) changes in hormone secretion/pulsatility, although it has been shown that acute resistance exercise does not affect the circadian rhythm of testosterone (Kraemer et al. 2001). Our analysis was conducted on blood samples that were collected at the midpoint of the training period and thus does not address how the acute hormone response may have changed over the course of the training period. However, we have recently reported similar acute hormone responses, and resting hormone concentrations, at the beginning and end of 15 weeks of training (West et al. 2009) and thus believe the measurements made at the midpoint represent a reasonable characterization of the acute hormonal milieu, an assumption shared by others (Ahtiainen et al. 2003a; Ronnestad et al. 2011). Our measures did not account for the various aggregate and splice variant isoforms of GH that are reported to be >100 in number (Kraemer et al. 2010).
Previously, associations between the acute increase in GH and fibre hypertrophy (McCall et al. 1999), and between pre- and post-training changes in acute testosterone responses and percentage of increase in quadriceps femoris CSA (Ahtiainen et al. 2003a), have been reported using small sample sizes (n = 11 and 7, respectively). In our view, it is difficult to interpret the correlations reported in these small data sets and their bearing on the importance of acute physiological growth hormone and testosterone responses to hypertrophy. Here we examined associations between the acute increases in GH, IGF-1, free testosterone, and cortisol, with adaptations to resistance training in 56 young men. Our sample had resistance training-induced gains in strength, LBM and muscle fibre CSA that represented a substantial range and that were normally distributed and therefore less prone to correlative bias due to outlying data points.
Acute changes in ostensibly anabolic hormones are frequently measured after resistance exercise with the assumption that they promote skeletal muscle anabolism (Ahtiainen et al. 2003b; Crewther et al. 2008; Gotshalk et al. 1997; Hakkinen and Pakarinen 1993; Kraemer et al. 1995, 2006; Migiano et al. 2009; Ronnestad et al. 2011). Because the biological roles of exercise-induced hormone changes are presently altogether uncertain, making concomitant measures of muscle (Spiering et al. 2009) or other tissues will provide insight into how the complex dynamic post-exercise hormonal milieu, which is a product of simultaneous secretion and clearance processes, might affect adaptation to exercise. Indeed, methods that measure hormone flux, in addition to characterizing the blood profile, will permit a clearer understanding of the actions of each hormone in producing a phenotypic change in response to training. For example, measurement of exercise-induced cortisol often presents a conundrum based on its equivocal physiological role. That is, cortisol is frequently elevated after resistance exercise protocols designed to elicit hypertrophy (Kraemer et al. 1993, 1998; Kraemer and Ratamess 2005) and yet is generally considered to be catabolic and as such counteractive to hypertrophy (Kraemer and Ratamess 2005; Spiering et al. 2008; Tarpenning et al. 2001). The findings of the present study are no different; that is, cortisol was significantly, albeit weakly, related to gains in type II fibre area. Likewise, GH was associated with changes in type I fibre hypertrophy and yet is reported to have no effect on myofibrillar protein accretion (Meinhardt et al. 2010; Rennie 2003; Yarasheski et al. 1993). As the regulation of GH becomes clearer (Inagaki et al. 2011), it is possible that a shared mechanism, such as neural drive/muscle activation and/or metabolic stress, that could affect both GH and muscle adaptation may explain the association of GH with hypertrophy. Collectively, GH and cortisol are known to have gluconeogenic action as well as liberate substrates such as free fatty acids (Sakharova et al. 2008) and amino acids (Simmons et al. 1984), respectively; it remains unknown whether exercise-induced changes in these hormones could also be modulating these energy-releasing and tissue-remodelling processes leading to an improved phenotype with training.
In contrast to GH, testosterone is known to have potent effects on contractile tissue accretion when administered pharmacologically (Bhasin et al. 1996). Due to the potency of exogenous testosterone for hypertrophy, physiological exercise-induced changes in testosterone have also garnered significant interest (Crewther et al. 2011; Hayes et al. 2010; Vingren et al. 2010). However, we did not observe any significant relationships between the exercise-induced increase in testosterone concentration and the degree of LBM, hypertrophy or strength. This observation is in agreement with our previous findings (West et al. 2009; Wilkinson et al. 2006) but is in contrast to a recent study (Ronnestad et al. 2011). The latter study (Ronnestad et al. 2011) reported that subjects who trained their elbow flexors after lower-body exercise, which elevated testosterone concentrations ~1.2-fold, achieved greater increases in strength and muscle CSA (at two of four scanned locations in the muscle mid-belly), despite equivalent progression in training load and equivalent increases in calculated muscle volume to elbow flexors trained alone. In the present study, free testosterone concentrations were elevated ~2-fold for ~15–30 min after lower-body exercise. In our view, the lack of association to adaptation was not surprising and is likely the result of the fact that exercise-induced testosterone elevations are small and transient compared to the sixfold increases that can be imposed by pharmacological intervention which are chronically sustained and which have potent effects on hypertrophy (Bhasin et al. 1996). Thus, our finding that the magnitude of the exercise-induced testosterone elevation was not associated with training adaptations may underscore differences between physiological and pharmacological interventions that affect muscle hypertrophy. Pharmacological administration of testosterone results in a different pattern (chronically elevated basal concentrations vs. transitory increases) and greater persistent increases in the concentration of the hormone (supraphysiological vs. physiological concentrations that are of comparable magnitude to the spread of normal diurnal variation).
In summary, differences in the post-exercise response free testosterone and IGF-1 showed no association with increases in adaptations to resistance training. While responses of GH and cortisol were positively correlated with changes in fibre area, the association was relatively weak and the relevance to hypertrophy is presently unclear due to evidence that GH is not anabolic to contractile tissue and that cortisol, which is catabolic in nature, is elevated after exercise programs that induce hypertrophy. A more detailed study of hormonal mechanisms is clearly required. Whereas increments in post-exercise GH and cortisol concentration were weakly associated with resistance training-induced phenotypes, other previously measured acute intramuscular markers, such as p70S6K1 phosphorylation, microRNA expression and satellite cell activation, can yield relatively robust associations with hypertrophy. Overall, because the regulation and biological actions of the post-exercise hormonal milieu are largely undefined, measuring systemic hormone profiles as well as local hormone and receptor concentrations, together with markers of hypertrophy or the phenotype itself, will enhance our understanding of their role in tissue remodelling with exercise.
References
Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Hakkinen K (2003a) Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol 89:555–563
Ahtiainen JP, Pakarinen A, Kraemer WJ, Hakkinen K (2003b) Acute hormonal and neuromuscular responses and recovery to forced vs maximum repetitions multiple resistance exercises. Int J Sports Med 24:410–418
Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM (2007) Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102:2232–2239
Beaven CM, Cook CJ, Gill ND (2008a) Significant strength gains observed in rugby players after specific resistance exercise protocols based on individual salivary testosterone responses. J Strength Cond Res 22:419–425
Beaven CM, Gill ND, Cook CJ (2008b) Salivary testosterone and cortisol responses in professional rugby players after four resistance exercise protocols. J Strength Cond Res 22:426–432
Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R (1996) The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335:1–7
Burd NA, Tang JE, Moore DR, Phillips SM (2009) Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 106:1692–1701
Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM (2010) Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588:3119–3130
Crewther B, Cronin J, Keogh J, Cook C (2008) The salivary testosterone and cortisol response to three loading schemes. J Strength Cond Res 22:250–255
Crewther BT, Cook C, Cardinale M, Weatherby RP, Lowe T (2011) Two emerging concepts for elite athletes: the short-term effects of testosterone and cortisol on the neuromuscular system and the dose-response training role of these endogenous hormones. Sports Med 41:103–123
Cribb PJ, Williams AD, Carey MF, Hayes A (2006) The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab 16:494–509
Curtin F, Schulz P (1998) Multiple correlations and bonferroni’s correction. Biol Psychiatry 44:775–777
Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM (2010) High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 110:309–317
Gotshalk LA, Loebel CC, Nindl BC, Putukian M, Sebastianelli WJ, Newton RU, Hakkinen K, Kraemer WJ (1997) Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol 22:244–255
Hakkinen K, Pakarinen A (1993) Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol 74:882–887
Hansen S, Kvorning T, Kjaer M, Sjogaard G (2001) The effect of short-term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports 11:347–354
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM (2007) Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86:373–381
Hayes LD, Bickerstaff GF, Baker JS (2010) Interactions of cortisol, testosterone, and resistance training: influence of circadian rhythms. Chronobiol Int 27:675–705
Inagaki Y, Madarame H, Neya M, Ishii N (2011) Increase in serum growth hormone induced by electrical stimulation of muscle combined with blood flow restriction. Eur J Appl Physiol 111:2715–2721
Judelson DA, Maresh CM, Yamamoto LM, Farrell MJ, Armstrong LE, Kraemer WJ, Volek JS, Spiering BA, Casa DJ, Anderson JM (2008) Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism, and metabolism. J Appl Physiol 105:816–824
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Kraemer WJ, Dziados JE, Marchitelli LJ, Gordon SE, Harman EA, Mello R, Fleck SJ, Frykman PN, Triplett NT (1993) Effects of different heavy-resistance exercise protocols on plasma beta-endorphin concentrations. J Appl Physiol 74:450–459
Kraemer WJ, Aguilera BA, Terada M, Newton RU, Lynch JM, Rosendaal G, McBride JM, Gordon SE, Hakkinen K (1995) Responses of IGF-I to endogenous increases in growth hormone after heavy-resistance exercise. J Appl Physiol 79:1310–1315
Kraemer WJ, Hakkinen K, Newton RU, McCormick M, Nindl BC, Volek JS, Gotshalk LA, Fleck SJ, Campbell WW, Gordon SE, Farrell PA, Evans WJ (1998) Acute hormonal responses to heavy resistance exercise in younger and older men. Eur J Appl Physiol Occup Physiol 77:206–211
Kraemer WJ, Loebel CC, Volek JS, Ratamess NA, Newton RU, Wickham RB, Gotshalk LA, Duncan ND, Mazzetti SA, Gomez AL, Rubin MR, Nindl BC, Hakkinen K (2001) The effect of heavy resistance exercise on the circadian rhythm of salivary testosterone in men. Eur J Appl Physiol 84:13–18
Kraemer RR, Hollander DB, Reeves GV, Francois M, Ramadan ZG, Meeker B, Tryniecki JL, Hebert EP, Castracane VD (2006) Similar hormonal responses to concentric and eccentric muscle actions using relative loading. Eur J Appl Physiol 96:551–557
Kraemer WJ, Dunn-Lewis C, Comstock BA, Thomas GA, Clark JE, Nindl BC (2010) Growth hormone, exercise, and athletic performance: a continued evolution of complexity. Curr Sports Med Rep 9:242–252
Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ (2009) Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587:211–217
McCall GE, Byrnes WC, Fleck SJ, Dickinson A, Kraemer WJ (1999) Acute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Can J Appl Physiol 24:96–107
Meinhardt U, Nelson AE, Hansen JL, Birzniece V, Clifford D, Leung KC, Graham K, Ho KK (2010) The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Ann Intern Med 152:568–577
Migiano MJ, Vingren JL, Volek JS, Maresh CM, Fragala MS, Ho JY, Thomas GA, Hatfield DL, Hakkinen K, Ahtiainen J, Earp JE, Kraemer WJ (2009) Endocrine response patterns to acute unilateral and bilateral resistance exercise in men. J Strength Cond Res 24:128–134
Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ (2005) Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288:E1153–E1159
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045
Oates BR, Glover EI, West DW, Fry JL, Tarnopolsky MA, Phillips SM (2010) Low-volume resistance exercise attenuates the decline in strength and muscle mass associated with immobilization. Muscle Nerve 42:539–546
Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM (2006) Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291:E937–E946
Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM (2008) Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104:1736–1742
Phillips SM (2004) Protein requirements and supplementation in strength sports. Nutrition 20:689–695
Phillips SM, Tang JE, Moore DR (2009) The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr 28:343–354
Rennie MJ (2003) Claims for the anabolic effects of growth hormone: a case of the emperor’s new clothes? Br J Sports Med 37:100–105
Ronnestad BR, Nygaard H, Raastad T (2011) Physiological elevation of endogenous hormones results in superior strength training adaptation. Eur J Appl Physiol 111:2249–2259
Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K, Barkan A (2008) Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab 93:2755–2759
Shepstone TN, Tang JE, Dallaire S, Schuenke MD, Staron RS, Phillips SM (2005) Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol 98:1768–1776
Simmons PS, Miles JM, Gerich JE, Haymond MW (1984) Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest 73:412–420
Spiering BA, Kraemer WJ, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Judelson DA, Joseph M, Vingren JL, Hatfield DL, Fragala MS, Ho JY, Maresh CM (2008) Effects of elevated circulating hormones on resistance exercise-induced Akt signaling. Med Sci Sports Exerc 40:1039–1048
Spiering BA, Kraemer WJ, Vingren JL, Ratamess NA, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Hakkinen K, Maresh CM (2009) Elevated endogenous testosterone concentrations potentiate muscle androgen receptor responses to resistance exercise. J Steroid Biochem Mol Biol 114:195–199
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 107:987–992
Tarpenning KM, Wiswell RA, Hawkins SA, Marcell TJ (2001) Influence of weight training exercise and modification of hormonal response on skeletal muscle growth. J Sci Med Sport 4:431–446
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E (2008) Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102:145–152
Tremblay MS, Copeland JL, Van HW (2004) Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol 96:531–539
Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM (2010) Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med 40:1037–1053
West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM (2009) Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol 108:60–67
Wilkinson SB, Tarnopolsky MA, Grant EJ, Correia CE, Phillips SM (2006) Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol 98:546–555
Yarasheski KE, Zachweija JJ, Angelopoulos TJ, Bier DM (1993) Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Physiol 74:3073–3076
Acknowledgments
Thanks to Dr. Jim Lyons for insight on statistical analyses. This work was supported by a Natural Science and Engineering Research Council (NSERC) of Canada grant to SMP. DWDW is a Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship Award recipient and all authors acknowledge those sources of support during the conduct of this research
Conflict of interest
The authors have no conflicts of interests to declare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fausto Baldissera.
Electronic supplementary material
Below is the link to the electronic supplementary material.
421_2011_2246_MOESM1_ESM.pdf
Online Resource 1. Subject physical characteristics, estimated dietary intakes, and single repetition strength pre- and post-training with data collapsed across groups for all subjects in (Hartman et al. 2007). (PDF 93 kb)
421_2011_2246_MOESM2_ESM.tif
Online Resource 2. Nutritional group (Control, Soy, Milk) responses of GH, free testosterone, IGF-1 and cortisol. Samples were drawn pre-exercise, immediately after exercise (RE—resistance exercise), and 15, 30, 60, and 90 min post-exercise. Values are means with upper and lower individual responses indicated. (TIFF 569 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
West, D.W.D., Phillips, S.M. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol 112, 2693–2702 (2012). https://doi.org/10.1007/s00421-011-2246-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2246-z