Abstract
Objectives
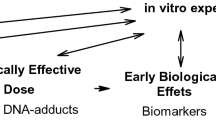
Urinary excretion of 2,5-hexanedione is currently used to estimate the exposure levels of hexane occurring to an individual during the previous work shift. However, because hexane exposures and urinary 2,5-hexanedione levels can vary considerably from day to day, and subchronic to chronic exposures to hexane are required to produce neuropathy, this biomarker may not accurately reflect the risk of an individual for developing hexane neuropathy. This investigation examines the potential of hexane-derived pyrrole adducts produced on globin and plasma proteins as markers for integrating cumulative exposures. Because the pyrrole markers incorporate bioactivation of hexane to 2,5-hexandione and the initial step of protein adduction involved in hexane-induced neuropathy, they potentially can serve as biomarkers of effect through reflecting pathogenetic events within the nervous system. Additionally, pyrrole formation is an irreversible reaction suggesting that hexane-derived protein pyrroles can be used to assess cumulative exposures to provide a better characterization of individual susceptibilities.
Methods
To examine the utility of the proposed markers, blood samples were obtained from eleven workers who used hexane for granulating metal powders in a slurry to produce metal machining die tools and four non-exposed volunteers. Globin and plasma were isolated, and the proteins were digested using pepsin, reacted with Ehrlich’s reagent and the level of pyrrole adducts were determined by absorbance at 530 nm. To determine the dose–response curve and dynamic range of the assay, erythrocytes were incubated with a range of 2,5-hexanedione concentrations and the net absorbance at 530 nm of isolated globin was measured.
Results
Pyrrole was detected in both the globin and plasma samples of the workers exposed to hexane and the levels of pyrroles in plasma were positively correlated with the levels of pyrroles in globin for most of the workers.
Conclusions
This investigation demonstrates that detectable levels of hexane-derived protein pyrrole adducts are produced on peripheral proteins following occupational exposures to hexane and supports the utility of measuring pyrroles for integrating cumulative exposures to hexane.







Similar content being viewed by others
References
Alexandar RS, Butler AR (1976) Electrophilic substitution in pyrroles. Part I. Reaction with 4-dimethylaminobenzaldehyde (Ehrlich’s reagent) in acid solution. J Chem Soc Perkin Trans 2:696–701
Boekelheide K (1987) 2,5-Hexanedione alters microtubule assembly. II. Enhanced polymerization of crosslinked tubulin. Toxicol Appl Pharmacol 88:383–396
DeCaprio AP (1986) Mechanisms of in vitro pyrrole adduct autoxidation in 2,5-hexanedione-treated protein. Mol Pharmacol 30:452–458
DeCaprio AP (1987) N-hexane neurotoxicity: a mechanism involving pyrrole adduct formation in axonal cytoskeletal protein. Neurotoxicology 8:199–210
DeCaprio AP, Olajos EJ, Weber P (1982) Covalent binding of a neurotoxic n-hexane metabolite: conversion of primary amines to substituted pyrrole adducts by 2,5-hexanedione. Toxicol Appl Pharmacol 65:440–450
DeCaprio AP, Strominger NL, Weber P (1983) Neurotoxicity and protein binding of 2,5-hexanedione in the hen. Toxicol Appl Pharmacol 68:297–307
DeCaprio AP, Briggs RG, Jackowski SJ, Kim JC (1988) Comparative neurotoxicity and pyrrole-forming potential of 2,5-hexanedione and perdeuterio-2,5-hexanedione in the rat. Toxicol Appl Pharmacol 92:75–85
DeCaprio AP, Kinney EA, LoPachin RM (2009) Comparative covalent protein binding of 2,5-hexanedione and 3-acetyl-2,5-hexanedione in the rat. J Toxicol Environ Health A 72:861–869
Filser JG, Peter H, Bolt HM, Fedtke N (1987) Pharmacokinetics of the neurotoxin n-hexane in rat and man. Arch Toxicol 60:77–80
Genter St Clair MB, Amarnath V, Moody MA, Anthony DC, Anderson CW, Graham DG (1988) Pyrrole oxidation and protein cross-linking as necessary steps in the development of gamma-diketone neuropathy. Chem Res Toxicol 1:179–185
Graham DG, Anthony DC, Boekelheide K, Maschmann NA, Richards RG, Wolfram JW et al (1982) Studies of the molecular pathogenesis of hexane neuropathy. II. Evidence that pyrrole derivatization of lysyl residues leads to protein crosslinking. Toxicol Appl Pharmacol 64:415–422
Graham DG, Anthony DC, Szakal-Quin G, Gottfried MR, Boekelheide K (1985) Covalent crosslinking of neurofilaments in the pathogenesis of n-hexane neuropathy. Neurotoxicology 6:55–63
Kawai T, Yasugi T, Mizunuma K, Horiguchi S, Uchida Y, Iwami O et al (1991) Dose-dependent increase in 2,5-hexanedione in the urine of workers exposed to n-hexane. Int Arch Occup Environ Health 63:285–291
Kessler W, Heilmaier H, Kreuzer P, Shen JH, Filser M, Filser JG (1990) Spectrophotometric determination of pyrrole-like substances in urine of rat and man: an assay for the evaluation of 2,5-hexanedione formed from n-hexane. Arch Toxicol 64:242–246
Mateus ML, dos Santos AP, Batoreu MC (2002) Evidence of zinc protection against 2,5-hexanedione neurotoxicity: correlation of neurobehavioral testing with biomarkers of excretion. Neurotoxicology 23:747–754
Sanz P, Flores IC, Soriano T, Repetto G, Repetto M (1995) In vitro quantitative structure-activity relationship assessment of pyrrole adducts production by gamma-diketone-forming neurotoxic solvents. Toxicol In Vitro 9:783–787
Shibata E, Huang J, Ono Y, Hisanaga N, Iwata M, Saito I et al (1990) Changes in urinary n-hexane metabolites by co-exposure to various concentrations of methyl ethyl ketone and fixed n-hexane levels. Arch Toxicol 64:165–168
Takahashi M, Takeuchi H, Kyo S, Yorifuji S, Seki Y, Hara I (1977) N-hexane polyneuropathy—a case report with a review of the literature. Med J Osaka Univ 28:77–85
Takeuchi Y, Ono Y, Hisanaga N, Kitoh J, Sugiura Y (1980) A comparative study on the neurotoxicity of n-pentane, n-hexane, and n-heptane in the rat. Br J Ind Med 37:241–247
Takeuchi Y, Ono Y, Hisanaga N, Kitoh J, Sugiura Y (1981) A comparative study of the toxicity of n-pentane, n-hexane, and n-heptane to the peripheral nerve of the rat. Clin Toxicol 18:1395–1402
Valentine WM, Amarnath V, Amarnath K, Erve JC, Graham DG, Morgan DL et al (1998) Covalent modification of hemoglobin by carbon disulfide: III. A potential biomarker of effect. Neurotoxicology 19:99–107
Yamada S (1967) Intoxication polyneuritis in the workers exposed to n-hexane. Sangyo Igaku 9:651–659
Yamamura Y (1969) N-hexane polyneuropathy. Folia Psychiatr Neurol Jpn 23:45–57
Yin H, Guo Y, Zeng T, Zhao X, Xie K (2013) Correlation between levels of 2, 5-hexanedione and pyrrole adducts in tissues of rats exposure to n-hexane for 5-days. PLoS One 8:e76011
Yin H, Guo Y, Song F, Zeng T, Xie K (2014a) Toxicokinetic study of pyrrole adducts and its potential application for biological monitoring of 2,5-hexanedione subacute exposure. Int Arch Occup Environ Health 87:655–662
Yin H, Zhang C, Guo Y, Shao X, Zeng T, Zhao X et al (2014b) Biological exposure indices of pyrrole adducts in serum and urine for hazard assessment of n-hexane exposure. PLoS One 9:e86108
Zhu M, Spink DC, Yan B, Bank S, DeCaprio AP (1994) Formation and structure of cross-linking and monomeric pyrrole autoxidation products in 2,5-hexanedione-treated amino acids, peptides, and protein. Chem Res Toxicol 7:551–558
Zhu M, Spink DC, Yan B, Bank S, DeCaprio AP (1995) Inhibition of 2,5-hexanedione-induced protein cross-linking by biological thiols: chemical mechanisms and toxicological implications. Chem Res Toxicol 8:764–771
Acknowledgements
Our sincere thanks to Dr. Doyle G. Graham (Vanderbilt University Medical Center, TN, USA), Dr. Yasutaka Ogawa, Dr. Naomi Hisanaga (National Institute of Industrial Health, Kanagawa, Japan) and Dr. Yasuhiro Takeuchi (Emeritus Professor of Nagoya University, Nagoya, Japan).
Author information
Authors and Affiliations
Contributions
GI and WMV deigned the project and wrote the manuscript. GI, VA and HLV measured pyrrole adducts and protein. TT, KM and TS conducted the field-work. TK measured urinary 2,5-hexanedione. VA drew the figure of pyrrole formation and detection.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
All assays were performed with the subjects’ informed consent following the Helsinki Declaration. The study protocol was approved by the ethical committee of Tokyo University of Science.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ichihara, G., Amarnath, V., Valentine, H.L. et al. Pyrrole adducts in globin and plasma of workers exposed to hexane. Int Arch Occup Environ Health 92, 873–881 (2019). https://doi.org/10.1007/s00420-019-01430-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-019-01430-7