Abstract
Objective
Recurrent seizures of autoimmune origin (AEp) are one of the most frequent causes of recurrent seizures or suspected epilepsy of unknown cause. The aim of this study was to identify specific phenotypes corresponding to AEp.
Methods
We retrospectively reviewed features of patients with recurrent seizures of unknown cause and investigated for suspected AEp (January 2015–May 2018). Patients were separated in: (1) AEpAb+: AEp with positive autoantibodies; (2) AEpAb−: suspected AEp (inflammatory central nervous system (CNS) profile) without autoantibodies; (3) NAEp: epilepsy without CNS inflammation.
Results
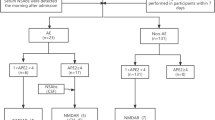
Eighty-nine epileptic patients underwent a CSF antibody detection. From the remaining 57 epileptic patients (32 excluded for a differential diagnosis), 61.4% were considered as AEp. 21% were AEpAb+ (4 NMDAR, 2 GABAbR, 3 GAD-Ab, 2 LGi1, 1 CASPR2), 40.4% AEpAb−, and 38.6% NAE. AE (AEpAb+ and AEpAb−) was significantly associated with antibody prevalence in epilepsy (APE) score ≥ 4 (80%), encephalitic phase (71.4%), psychiatric involvement (64.7%), cognitive impairment (50%), and status epilepticus (41.2%). Within the group of 29 patients without encephalitic phase and with chronic epilepsy (NEPp), 34.5% were defined as AEp. 10.4% were AEpAb+ (2 GAD, 1 CASPR2) and 24.1% were AEpAb−. NEP AEp was associated with non-cerebral autoimmune disorders, short epileptic disease duration, and cognitive impairment.
Conclusions
Autoimmune cause (AEp) should be assessed in patient suffering from recurrent seizures of unknown cause. Acute encephalitis is clearly the main AEp phenotype. AEp was also defined in more than one-third of chronic epilepsy patients (NEP) of unknown cause. Then, AEp may be combined with other autoimmune comorbidities, a shorter evolution of recurrent seizures, and cognitive impairment.



Similar content being viewed by others
References
Hauser WA, Annegers JF, Rocca WA (1996) Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 71(6):576–586
Annegers JF, Rocca WA, Hauser WA (1996) Causes of epilepsy: contributions of the Rochester epidemiology project. Mayo Clin Proc 71(6):570–575
Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342(5):314–319
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M et al (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7(12):1091–1098
Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P et al (2016) Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain 139(Pt 4):1079–1093
Brenner T, Sills GJ, Hart Y, Howell S, Waters P, Brodie MJ et al (2013) Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia 54(6):1028–1035
Wright S, Vincent A (2016) Progress in autoimmune epileptic encephalitis. Curr Opin Neurol 29(2):151–157
Dubey D, Alqallaf A, Hays R, Freeman M, Chen K, Ding K et al (2017) Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol 74(4):397–402
Tecellioglu M, Kamisli O, Kamisli S, Yucel FE, Ozcan C (2018) Neurological autoantibodies in drug-resistant epilepsy of unknown cause. Ir J Med Sci 187(4):1057–1063
Soeder BM, Gleissner U, Urbach H, Clusmann H, Elger CE, Vincent A et al (2009) Causes, presentation and outcome of lesional adult onset mediotemporal lobe epilepsy. J Neurol Neurosurg Psychiatry 80(8):894–899
Gozubatik-Celik G, Ozkara C, Ulusoy C, Gunduz A, Delil S, Yeni N et al (2017) Anti-neuronal autoantibodies in both drug responsive and resistant focal seizures with unknown cause. Epilepsy Res 135:131–136
Gaspard N, Foreman BP, Alvarez V, Kang CC, Probasco JC, Jongeling AC et al (2015) New-onset refractory status epilepticus. Neurology 85(18):1604–1613
Süße M, Hamann L, Flöel A, von Podewils F (2019) Nonlesional late-onset epilepsy: semiology, EEG, cerebrospinal fluid, and seizure outcome characteristics. Epilepsy Behav 91:75–80
von Podewils F, Suesse M, Geithner J, Gaida B, Wang Z, Lange J et al (2017) Prevalence and outcome of late-onset seizures due to autoimmune etiology: a prospective observational population-based cohort study. Epilepsia 58(9):1542–1550
Vanli-Yavuz EN, Erdag E, Tuzun E, Ekizoglu E, Baysal-Kirac L, Ulusoy C et al (2016) Neuronal autoantibodies in mesial temporal lobe epilepsy with hippocampal sclerosis. J Neurol Neurosurg Psychiatry 87(7):684–692
Steriade C, Britton J, Dale RC, Godot A, Irani S, Linnoila J et al (2020) Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune associated epilepsy: conceptual definitions. Epilepsia 61:1341–1351
Joubert B, Saint-Martin M, Noraz N, Picard G, Rogemond V, Ducray F et al (2016) Characterization of a subtype of autoimmune encephalitis with anti–contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol 73(9):1115–1124
Thompson J, Bi M, Murchison AG, Makuch M, Bien CG, Chu K et al (2018) The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141(2):348–356
Bermeo-Ovalle A (2017) Scratching the surface in autoimmune epilepsy: it is the time to dig deeper, but how? Epilepsy Curr 17(4):225–226
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404
Graus F, Escudero D, Oleaga L, Bruna J, Villarejo-Galende A, Ballabriga J et al (2018) Syndrome and outcome of antibody-negative limbic encephalitis. Eur J Neurol 25(8):1011–1016
Graus F, Saiz A, Lai M, Bruna J, Lopez F, Sabater L et al (2008) Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology 71(12):930–936
Dubey D, Singh J, Britton JW, Pittock SJ, Flanagan EP, Lennon VA et al (2017) Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia 58(7):1181–1189
Tekturk P, Baykan B, Erdag E, Peach S, Sezgin M, Yapici Z et al (2018) Investigation of neuronal auto-antibodies in children diagnosed with epileptic encephalopathy of unknown cause. Brain Dev 40(10):909–917
Modoni A, Masciullo M, Spinelli P, Marra C, Tartaglione T, Andreetta F et al (2009) Successful treatment of acute autoimmune limbic encephalitis with negative VGKC and NMDAR antibodies. Cogn Behav Neurol 22(1):63–66
Najjar S, Pearlman D, Zagzag D et al (2011) Spontaneously resolving seronegative autoimmune limbic encephalitis. Cogn Behav Neurol 24(2):99–105
Najjar S, Pearlman D, Devinsky O, Devinsky O (2013) Neuropsychiatric autoimmune encephalitis without VGKC-complex, NMDAR, and GAD autoantibodies: case report and literature review. Cogn Behav Neurol 26(1):36–49
Toro J, Cuellar-Giraldo D, Duque A, Minota K, Patino J, Garcia M (2017) Seronegative paraneoplastic limbic encephalitis associated with thymoma. Cogn Behav Neurol 30(3):125–128
Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J (2000) Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 123(Pt 7):1481–1494
Ahmad SAB, Archer HA, Rice CM, Gerhand S, Bradley M, Wilkins A (2011) Seronegative limbic encephalitis: case report, literature review and proposed treatment algorithm. Pract Neurol 11(6):355–361
Fisher RE, Patel NR, Lai EC, Schulz PE (2012) Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med 37(9):e213-218.32
Gaspard N (2016) Autoimmune epilepsy. Continuum 22(1 Epilepsy):227–245
Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J et al (2010) Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 9(1):67–76
Daif A, Lukas RV, Issa NP, Javed A, VanHaerents S, Reder AT et al (2018) Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy Behav 80:331–336
Gagnon M-M, Savard M (2016) Limbic encephalitis associated with GAD65 antibodies: brief review of the relevant literature. Can J Neurol Sci 43(4):486–493
Falip M, Carreño M, Miró J, Saiz A, Villanueva V, Quilez A et al (2012) Prevalence and immunological spectrum of temporal lobe epilepsy with glutamic acid decarboxylase antibodies. Eur J Neurol 19(6):827–833
Sunwoo J-S, Lee S-T, Byun J-I, Moon J, Shin J-W, Jeong D-E et al (2015) Clinical manifestations of patients with CASPR2 antibodies. J Neuroimmunol 15(281):17–22
Chen C, Wang X, Zhang C, Tao C, Shi W-X, Guan H-Z et al (2017) Seizure semiology in leucine-rich glioma-inactivated protein 1 antibody-associated limbic encephalitis. Epilepsy Behav 77:90–95
Ong M-S, Kohane IS, Cai T, Gorman MP, Mandl KD (2014) Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol 1(5):569–574
Lopez-Sublet M, Bihan H, Reach G, Dupont S, Didelot A, Mourad J-j et al (2012) Limbic encephalitis and type 1 diabetes with glutamic acid decarboxylase 65 (GAD65) autoimmunity: improvement with high-dose intravenous immunoglobulin therapy. Diabetes Metab 38(3):273–275
Author information
Authors and Affiliations
Contributions
MG and LT: designed and conceptualized the study; acquisition of data; analyzed the data; draft the manuscript for intellectual content. SF and LH: designed and conceptualized the study; interpreted the data; revised the manuscript for intellectual content. AV, BJ, and JH: interpreted the data; revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard statement
This study was conducted in accordance with the ethical standards recognized internationally.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goudot, M., Frismand, S., Hopes, L. et al. Recurrent seizures of autoimmune origin: emerging phenotypes. J Neurol 268, 3000–3010 (2021). https://doi.org/10.1007/s00415-021-10457-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10457-1