Abstract
Background
Differential diagnosis of neurodegenerative dementia is currently supported by biomarkers including cerebrospinal fluid (CSF) tests. Among them, CSF total-tau (t-tau), phosphorylated tau (p-tau) and β-amyloid42 (Aβ42) are considered core biomarkers of neurodegeneration. In the present work, we hypothesize that simultaneous assessment of these biomarkers together with CSF α-synuclein (α-syn) will significantly improve the differential diagnostic of Alzheimer’s disease and other dementias. To that aim, we characterized the analytical and clinical performance of a new tetra-plex immunoassay that simultaneously quantifies CSF Aβ42, t-tau, p-tau and α-syn in the differential diagnosis of neurodegenerative dementia.
Methods
Biomarkers’ concentrations were measured in neurological controls (n = 38), Alzheimer’s disease (n = 35), Creutzfeldt–Jakob disease (n = 37), vascular dementia (n = 28), dementia with Lewy bodies/Parkinson’s disease dementia (n = 27) and frontotemporal dementia (n = 34) using the new tetra-plex assay and established single-plex assays. Biomarker’s performance was evaluated and diagnostic accuracy in the discrimination of diagnostic groups was determined using partial least squares discriminant analysis.
Results
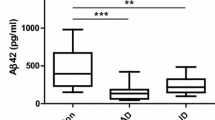
The tetra-plex assay presented accuracies similar to individual single-plex assays with acceptable analytical performance. Significant correlations were observed between tetra-plex and single-plex assays. Using partial least squares discriminant analysis, Alzheimer’s disease and Creutzfeldt–Jakob disease were well differentiated, reaching high accuracies in the discrimination from the rest of diagnostic groups.
Conclusions
The new tetra-plex assay coupled with multivariate analytical approaches becomes a valuable asset for the differential diagnosis of neurodegenerative dementia and related applications.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- AD:
-
Alzheimer’s disease
- CJD:
-
Creutzfeldt–Jakob disease
- t-tau:
-
Total-tau
- p-tau:
-
Phosphorylated tau
- Aβ42:
-
β-Amyloid42
- RT-QuIC:
-
Real-time quacking-induced conversion
- VaD:
-
Vascular dementia
- LBD:
-
Lewy body diseases
- FTD:
-
Fronto-temporal dementia
- α-syn:
-
α-Synuclein
- ND:
-
Neurological controls
- DLB:
-
Dementia with Lewy bodies
- PDD:
-
Parkinson’s disease dementia
- bvFTD:
-
Behavioral variant FTD
- LM:
-
Linear models
- LRT:
-
Likelihood ratio tests
- AUCs:
-
Areas under the curve
- 95% CI:
-
95% Confidence intervals
- ROC:
-
Receiver operating characteristic
- PLS-DA:
-
Partial least squares discriminant analysis
- CV:
-
Intra-coefficient of variation
- RT:
-
Room temperature
- F/T:
-
Freeze/thawing
- PCA:
-
Principal component analysis
- VIP:
-
Variable importance for the projection
- SD:
-
Standard deviation
- f:
-
Female
- m:
-
Male
References
Armstrong RA, Lantos PL, Cairns NJ (2005) Overlap between neurodegenerative disorders. Neuropathology 25:111–124
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement [Internet] 7(3):263–269. https://www.ncbi.nlm.nih.gov/pubmed/21514250%5Cn, https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3312024
Hermann P, Laux M, Glatzel M, Matschke J, Knipper T, Goebel S et al (2018) Validation and utilization of amended diagnostic criteria in Creutzfeldt–Jakob disease surveillance. Neurology. https://www.neurology.org/lookup/doi/10.1212/WNL.0000000000005860
Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132(Pt 10):2659–2668
Schmitz M, Ebert E, Stoeck K, Karch A, Collins S, Calero M et al (2016) Validation of 14-3-3 protein as a marker in sporadic Creutzfeldt–Jakob disease diagnostic. Mol Neurobiol 53(4):2189–2199
Cramm M, Schmitz M, Karch A, Mitrova E, Kuhn F, Schroeder B et al (2016) Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt–Jakob disease. Mol Neurobiol 53(3):1896–1904
Llorens F, Schmitz M, Karch A, Cramm M, Lange P, Gherib K et al (2016) Comparative analysis of cerebrospinal fluid biomarkers in the differential diagnosis of neurodegenerative dementia. Alzheimer’s Dement 12(5):577–589
Schoonenboom NSM, Reesink FE, Verwey NA, Kester MI, Teunissen CE, Van De Ven PM et al (2012) Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78(1):47–54
Bruun M, Rhodius-Meester HFM, Koikkalainen J, Baroni M, Gjerum L, Lemstra AW et al (2018) Evaluating combinations of diagnostic tests to discriminate different dementia types. Alzheimer’s Dement Diagn Assess Dis Monit 10:509–518
Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B et al (2005) Simultaneous measurement of β-amyloid(1–42), total Tau, and phosphorylated Tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem 51(2):336–345
Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ et al (2011) Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1–42, Total tau, and P-tau181 for identifying alzheimer disease amyloid plaque pathology. Arch Neurol 68(9):1137–1144
Vanderstichele H, De Meyer G, Andreasen N, Kostanjevecki V, Wallin A, Olsson A et al (2005) Amino-truncated β-amyloid 42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin Chem 51(9):1650–1660
Lim X, Yeo JM, Green A, Pal S (2013) The diagnostic utility of cerebrospinal fluid alpha-synuclein analysis in dementia with Lewy bodies—a systematic review and meta-analysis. Parkinsonism Relat Disord 19:851–858
Zhou B, Wen M, Yu WF, Zhang CL, Jiao L (2015) The diagnostic and differential diagnosis utility of cerebrospinal fluid α-synuclein levels in Parkinson’s disease: a meta-analysis. Parkinsons Dis. https://doi.org/10.1155/2015/567386
Llorens F, Kruse N, Schmitz M, Gotzmann N, Golanska E, Thüne K et al (2016) Evaluation of α-synuclein as a novel cerebrospinal fluid biomarker in different forms of prion diseases. Alzheimer’s Dement [Internet]. https://linkinghub.elsevier.com/retrieve/pii/S1552526016330552
Llorens F, Kruse N, Karch A, Schmitz M, Zafar S, Gotzmann N et al (2017) Validation of α-synuclein as a CSF biomarker for sporadic Creutzfeldt–Jakob disease. Mol Neurobiol [Internet]. https://link.springer.com/10.1007/s12035-017-0479-5
Oeckl P, Metzger F, Nagl M, von Arnim CAF, Halbgebauer S, Steinacker P et al (2016) Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer’s and Creutzfeldt–Jakob disease but no alteration in synucleinopathies. Mol Cell Proteomics [Internet] 15(10):3126–3138. https://www.mcponline.org/lookup/doi/10.1074/mcp.M116.059915
Schmitz M, Villar-Piqué A, Llorens F, Gmitterová K, Hermann P, Varges D et al (2019) Cerebrospinal fluid total and phosphorylated α-synuclein in patients with Creutzfeldt–Jakob disease and synucleinopathy. Mol Neurobiol 56(5):3476–3483
Shi M, Tang L, Toledo JB, Ginghina C, Wang H, Aro P et al (2018) Cerebrospinal fluid α-synuclein contributes to the differential diagnosis of Alzheimer’s disease. Alzheimer’s Dement 14(8):1052–1062
Chiasserini D, Biscetti L, Eusebi P, Salvadori N, Frattini G, Simoni S et al (2017) Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimer’s Res Ther 9(1):52
Twohig D, Rodriguez-Vieitez E, Sando SB, Berge G, Lauridsen C, Møller I et al (2018) The relevance of cerebrospinal fluid α-synuclein levels to sporadic and familial Alzheimer’s disease. Acta Neuropathol Commun 6(1):130
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(9):2456–2477
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D et al (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89(1):88–100. https://www.ncbi.nlm.nih.gov/pubmed/28592453%0A, https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5496518
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B et al (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80(5):496–503
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32(6):853–864
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH et al (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 43(2):250–260
Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132(10):2659–2668
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 12
Passing H, Bablok W (1983) A new biometrical procedure for testing the equality of measurements from two different analytical methods. Clin Chem Lab Med [Internet] 21(11):709–720. https://www.degruyter.com/view/j/cclm.1983.21.issue-11/cclm.1983.21.11.709/cclm.1983.21.11.709.xml
Pérez-Enciso M, Tenenhaus M (2003) Prediction of clinical outcome with microarray data: a partial least squares discriminant analysis (PLS-DA) approach. Hum Genet [Internet] 112(5–6):581–592. https://www.ncbi.nlm.nih.gov/pubmed/12607117
Services USD of H and H (2018) Guidance for industry: bioanalytical method validation guidance for industry bioanalytical method validation. US Food Drug Adm [Internet], 1–22. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B et al (2005) Simultaneous measurement of β-amyloid (1–42), total Tau, and phosphorylated Tau (Thr 181) in cerebrospinal fluid by the xMAP technology. Clin Chem 51(2):336–345
Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S et al (2011) The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer’s Dement 7(4):386–395
Leitão MJ, Silva-Spínola A, Santana I, Olmedo V, Nadal A, Le Bastard N et al (2019) Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer’s disease. Alzheimer’s Res Ther 11(1)
Kollhoff AL, Howell JC, Hu WT (2018) Automation vs. experience: Measuring Alzheimer’s beta-amyloid 1–42 peptide in the CSF. Front Aging Neurosci 10(AUG)
Lifke V, Kollmorgen G, Manuilova E, Oelschlaegel T, Hillringhaus L, Widmann M et al (2019) Elecsys® Total-Tau and Phospho-Tau (181P) CSF assays: Analytical performance of the novel, fully automated immunoassays for quantification of tau proteins in human cerebrospinal fluid. Clin Biochem 72:30–38
Toombs J, Paterson RW, Schott JM, Zetterberg H (2014) Amyloid-beta 42 adsorption following serial tube transfer. Alzheimer’s Res Ther 6(1)
del Campo M, Mollenhauer B, Bertolotto A, Engelborghs S, Hampel H, Simonsen AH et al (2012) Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: an update. Biomark Med 6(4):419–430
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7(3):263–269
Reijn TSM, Rikkert MO, Van Geel WJA, De Jong D, Verbeek MM (2007) Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid β42 and tau proteins. Clin Chem 53(5):859–865
Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Sáanchez-Valle R, Mitrováa E et al (2006) CSF tests in the differential diagnosis of Creutzfeldt–Jakob disease. Neurology 67(4):637–643
Kruse N, Heslegrave A, Gupta V, Foiani M, Villar-Piqué A, Schmitz M et al (2018) Interlaboratory validation of cerebrospinal fluid α-synuclein quantification in the diagnosis of sporadic Creutzfeldt–Jakob disease. Alzheimer’s Dement Diagn Assess Dis Monit 10:461–470
Lim X, Yeo JM, Green A, Pal S (2013) The diagnostic utility of cerebrospinal fluid alpha-synuclein analysis in dementia with Lewy bodies—a systematic review and meta-analysis. Parkinsonism Relat Disord [Internet] 19(10):851–858. https://www.ncbi.nlm.nih.gov/pubmed/23886935
Schmitz M, Villar-Piqué A, Llorens F, Gmitterová K, Hermann P, Varges D et al (2018) Cerebrospinal fluid total and phosphorylated α-synuclein in patients with Creutzfeldt–Jakob disease and synucleinopathy. Mol Neurobiol 56:3476–3483
Majbour NK, Chiasserini D, Vaikath NN, Eusebi P, Tokuda T, Van De Berg W et al (2017) Increased levels of CSF total but not oligomeric or phosphorylateed forms of alpha-synuclein in patients diagnosed with probable Alzheimer’s disease. Sci Rep 7
Llorens F, Kruse N, Schmitz M, Gotzmann N, Golanska E, Thüne K et al (2017) Evaluation of α-synuclein as a novel cerebrospinal fluid biomarker in different forms of prion diseases. Alzheimer’s Dement 13(6):710–719
Lattanzio F, Abu-Rumeileh S, Franceschini A, Kai H, Amore G, Poggiolini I et al (2017) Prion-specific and surrogate CSF biomarkers in Creutzfeldt–Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 133(4):559–578
Lehmann S, Paquet C, Malaplate-Armand C, Magnin E, Schraen S, Quillard-Muraine M et al (2019) Diagnosis associated with Tau higher than 1200 pg/mL: insights from the clinical and laboratory practice. Clin Chim Acta [Internet]. https://linkinghub.elsevier.com/retrieve/pii/S0009898119318406
Zerr I, Schmitz M, Karch A, Villar-Piqué A, Kanata E, Golanska E et al (2018) Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimer’s Dement [Internet]. https://www.ncbi.nlm.nih.gov/pubmed/29391125%0A, https://linkinghub.elsevier.com/retrieve/pii/S1552526018300025
Landqvist Waldö M, Frizell Santillo A, Passant U, Zetterberg H, Rosengren L, Nilsson C et al (2013) Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol [Internet] 13(1):54. https://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-13-54
Funding
This study was funded by the Instituto Carlos III (Grants CP/00041 and PI19/00144) and by the Fundació La Marató de TV3 (201821-30-31-32) to FL and by the Robert Koch Institute through funds from the Federal Ministry of Health (Grant no. 1369-341) to IZ. JADR received funding from MICINN (RTI2018-099773-B-100) and La Caixa Banking Foundation under the project code HR18-00452. This project was also funded at 65% by the Fondo Europeo de Desarrollo Regional (FEDER) through the Interreg V-A-Spain-France-Andorra (POCTEFA 2014–2020) programme. AVP is supported by the Beatriu de Pinós programme (2018-BP-00129) from the Ministry of Business and Knowledge of the Government of Catalonia, cofunded by the EU Horizon 2020 programme under an MSCA grant agreement (801370). We thank the CERCA Programme of Generalitat de Catalunya for institutional support.
Author information
Authors and Affiliations
Contributions
FL designed the study. DD-L, GE, AV-P, JADR, EM, IF and FL participated in the acquisition and analysis of data. PH, MS, IS, IB, IZ and FL collected and characterized biological samples and contributed to data interpretation. GE and FL drafted the manuscript and the figures. All authors critically revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
Written informed consent was obtained from all study participants or their legal guardians. The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by all local Ethics committees (Reference numbers 11/11/93, 5/09/08, 9/06/08, 19/11/09, Universitätsmedizin Göttingen, Germany and HUC-43-09, University of Coimbra, Portugal).
Electronic supplementary material
Supplementary Figure 1. Analytical performance of new tetra-plex assay for quantification of CSF Aβ42, t-tau, p-tau and α-syn. (a) Analytical performance of the tetra-plex assay. Intra and inter coefficients of variation (CV), lower limit of quantification (LLOQ) and upper limit of quantification (ULOQ) and linearity are indicated for Aβ42, t-tau, p-tau and α-syn. (b) Representative standard curves for each biomarker.
Supplementary Figure 2. Comparison on diagnostic performance between tetra-plex (fluorimetric) and single-plex (colorimetric) assays. Area Under the Curve (AUC) derived from receiver operating characteristic curves with 95% confidence interval for the comparison for the tetra-plex and single-plex assays. Accuracy was calculated for the four biomarkers (Aβ42, t-tau, p-tau and α-syn) in the comparison among neurodegenerative dementias (AD, Alzheimer’s disease; CJD, Creutzfeldt-Jakob disease; VaD, vascular dementia; DLB/PDD, dementia with Lewy bodies and Parkinson’s disease dementia; FTD, frontotemporal dementia). Statistical differences between AUCs derived from tetra-plex and single-plex assays is indicated, with statistically significant values (considered as p<0.05) highlighted in bold.
Supplementary Figure 3. Patient outlier detection in the agreement analysis of tetra-plex and single-plex assays based on Cook’s distance.
Supplementary Figure 4. Unsupervised classification of ND, AD and CJD patients based on Principal Component Analysis (PCA). (a) First two PCA components involving t-tau, p-tau, α-syn, and Aβ42. (b) First two PCA components of t-tau, p-tau and Aβ42 in the absence of α-syn.
Supplementary Table 1. Sensitivities and specificities associated to the AUC comparisons between ND and neurodegenerative dementias. Sensitivities and specificities in % based in Youden index are indicated for each comparison for the four biomarkers in the tetra-plex and single-plex assays.
Supplementary Table 2. Diagnostic accuracy of the tetra-plex assay using PLS-DA in the discrimination of AD. PLS-DAs were constructed based on training datasets. Variable importance for the projection (VIP) criterion was used to identify which biomarkers contribute most on the classification performance. VIP scores estimate the contribution of each biomarker in the in the PLS-DA model, according to the variance explained by each PLS component. A biomarker with a VIP score close to or greater than 1 is considered important in the given model. Accuracy, sensitivity and specificity diagnostic measures are indicated. The training and test sets random partitions were generated 1000 times and statistical summaries (median, 2.5th and 97.5th quintiles, termed here 95% Confidence Interval) were computed for each diagnostic measure. Accuracies with random non informative data were obtained based on a permutation test involving 1000 data sets constructed by randomly reassigning class labels at each individual, then performing a PLS-DA on the new randomized training data sets and computing diagnostic measures in their respective 1000 randomized test sets. AD, Alzheimer’s disease; VaD, vascular dementia; DLB/PDD, dementia with Lewy bodies and Parkinson’s disease dementia; FTD, frontotemporal dementia. Aβ42, β-amyloid42; t-tau, total-tau, p-tau, phospho-tau and α-syn, α-synuclein.
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diaz-Lucena, D., Escaramis, G., Villar-Piqué, A. et al. A new tetra-plex fluorimetric assay for the quantification of cerebrospinal fluid β-amyloid42, total-tau, phospho-tau and α-synuclein in the differential diagnosis of neurodegenerative dementia. J Neurol 267, 2567–2581 (2020). https://doi.org/10.1007/s00415-020-09870-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09870-9