Abstract
Background
We investigated the relationship between three language-dependent behaviors (i.e., command-following, intelligible verbalization, and intentional communication) and the functional status of patients with disorders of consciousness (DoC). We hypothesized that patients in minimally conscious state (MCS) who retain behavioral evidence of preserved language function would have similar levels of functional disability, while patients who lack these behaviors would demonstrate significantly greater disability. We reasoned that these results could then be used to establish empirically-based diagnostic criteria for MCS+.
Methods
In this retrospective cohort study we included rehabilitation inpatients diagnosed with DoC following severe-acquired brain injury (MCS = 57; vegetative state/unresponsive wakefulness syndrome [VS/UWS] = 63); women: 46; mean age: 47 ± 19 years; traumatic etiology: 68; time post-injury: 40 ± 23 days). We compared the scores of the Disability Rating Scale score (DRS) at time of transition from VS/UWS to MCS or from MCS– to MCS+, and at discharge between groups.
Results
Level of disability on the DRS was similar in patients with any combination of the three language-related behaviors. MCS patients with no behavioral evidence of language function (i.e., MCS–) were more functionally impaired than patients with MCS+ at time of transition and at discharge.
Conclusions
Command-following, intelligible verbalization, and intentional communication are not associated with different levels of functional disability. Thus, the MCS+ syndrome can be diagnosed based on the presence of any one of these language-related behaviors. Patients in MCS+ may evidence less functional disability compared to those in MCS who fail to demonstrate language function (i.e., MCS–).




Similar content being viewed by others
Change history
19 March 2020
Following electronic publication of the above-referenced manuscript, we discovered that one of the three criteria we proposed to establish command-following in the MCS+ syndrome was inadvertently omitted in some parts of the manuscript.
Abbreviations
- CRS-R:
-
Coma recovery scale-revised
- DoC:
-
Disorders of consciousness
- DRS:
-
Disability rating scale
- MCS:
-
Minimally conscious state
- TBI:
-
Traumatic brain injury
- UWS/VS:
-
Unresponsive wakefulness syndrome/vegetative state
References
Demertzi A, Ledoux D, Bruno MA et al (2011) Attitudes towards end-of-life issues in disorders of consciousness: a European survey. J Neurol 258:1058–1065. https://doi.org/10.1007/s00415-010-5882-z
Stender J, Gosseries O, Bruno MA et al (2014) Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 384:514–522. https://doi.org/10.1016/S0140-6736(14)60042-8
Di Perri C, Thibaut A, Heine L et al (2016) Towards new methods of diagnosis in disorders of consciousness—authors’ reply. Lancet Neurol 15:1115–1116
Gosseries O, Zasler ND, Laureys S (2014) Recent advances in disorders of consciousness: focus on the diagnosis. Brain Inj 28:1141–1150. https://doi.org/10.3109/02699052.2014.920522
The Multi-Society Task Force on PVS (1994) Medical aspects of the persistent vegetative state (1). The Multi-Society Task Force on PVS. N Engl J Med 330:1499–1508. https://doi.org/10.1056/NEJM199405263302107
Laureys S, Celesia GG, Cohadon F et al (2010) Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 8:68. https://doi.org/10.1186/1741-7015-8-68
Giacino JT, Ashwal S, Childs N et al (2002) The minimally conscious state. Neurology 58:349–353
Giacino JT, Kalmar K, Whyte J (2004) The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85:2020–2029
Bruno MA, Vanhaudenhuyse A, Thibaut A et al (2011) From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 258:1373–1384
Bruno M-A, Majerus S, Boly M et al (2012) Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol 259:1087–1098. https://doi.org/10.1007/s00415-011-6303-7
Zheng ZS, Reggente N, Lutkenhoff E et al (2017) Disentangling disorders of consciousness: Insights from diffusion tensor imaging and machine learning. Hum Brain Mapp 38:431–443. https://doi.org/10.1002/hbm.23370
Aubinet C, Larroque SK, Heine L et al (2018) Clinical subcategorization of minimally conscious state according to resting functional connectivity. Hum Brain Mapp 39:4519–4532
Guldenmund P, Soddu A, Baquero K et al (2016) Structural brain injury in patients with disorders of consciousness: a voxel-based morphometry study. Brain Inj 30:343–352. https://doi.org/10.3109/02699052.2015.1118765
Estraneo A, Loreto V, Guarino I et al (2016) Standard EEG in diagnostic process of prolonged disorders of consciousness. Clin Neurophysiol 127:2379–2385. https://doi.org/10.1016/j.clinph.2016.03.021
Schnakers C, Edlow BL, Chatelle C, Giacino JT (2015) Minimally conscious state. In: Laureys S, Gosseries O, Tononi G (eds) The neurology of consciousness. Academic Press, Cambridge
Rappaport M, Hall KM, Hopkins K et al (1982) Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil 63:118–123
Giacino JT, Kalmar K, Whyte J (2004) The JFK Coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85:2020–2029
Seel RT, Sherer M, Whyte J et al (2010) Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch Phys Med Rehabil 91:1795–1813. https://doi.org/10.1016/j.apmr.2010.07.218
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Longoni F, Grande M, Hendrich V et al (2005) An fMRI study on conceptual, grammatical, and morpho-phonological processing. Brain Cogn 57:131–134. https://doi.org/10.1016/j.bandc.2004.08.032
Vigneau M, Beaucousin V, Herve PY et al (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30:1414–1432
Davis MH, Johnsrude IS (2003) Hierarchical processing in spoken language comprehension. J Neurosci 23:3423–3431
Hickok G, Poeppel D (2007) The cortical organization of speech processing. Nat Rev Neurosci 8:393–402
Riecker A, Mathiak K, Wildgruber D et al (2005) fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology 64:700–706. https://doi.org/10.1212/01.WNL.0000152156.90779.89
Gabrieli JD, Poldrack RA, Desmond JE (1998) The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA 95:906–913
Willems RM, Varley R (2010) Neural insights into the relation between language and communication. Front Hum Neurosci 4:1–8. https://doi.org/10.3389/fnhum.2010.00203
Tomasello M (2008) Origins of human communication. MIT Press, Cambridge
Tomasello M, Carpenter M, Call J et al (2005) In Search of the uniquely human. Behav Brain Sci 28:721–727. https://doi.org/10.1017/S0140525X05540123
Airenti G (2010) Is a naturalistic theory of communication possible? Cogn Syst Res 11:165–180. https://doi.org/10.1016/j.cogsys.2009.03.002
Bara BG (2011) Cognitive pragmatics: the mental processes of communication. Intercult Pragmat 8:443–485
Goodwin C (1995) Co-constructing meaning in conversations with an Aphasie man. Res Lang Soc Interact 28:233–260. https://doi.org/10.1207/s15327973rlsi2803_4
Bara B, Cutica I, Tirassa M (2001) Neuropragmatics: extralinguistic communication after closed head injury. Brain Lang 77:72–94. https://doi.org/10.1006/brln.2000.2430
Acknowledgements
Dr. Aurore Thibaut is a FNRS is a post-doctoral research fellow and has been supported by the Wallonie Brussel International (WBI) scholarship, the Belgian American Educational Foundation (BAEF), and the Leon Fredericq Foundation. Dr. Bodien is supported by the National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR), Administration for Community Living (90DP0039, Spaulding-Harvard TBI Model System). Dr. Giacino received support from NIDILRR (90DP0039, Spaulding-Harvard TBI Model System) and the James S. McDonnell Foundation (Understanding Human Cognition-Collaborative). The authors thank the clinical staff at Spaulding Hospital Cambridge and Spaulding Rehabilitation Hospital for acquiring the clinical metrics used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflict of interest.
Ethical standards
The REDCap database was approved by the local Institutional Review Board. Data were acquired during routine clinical care by trained clinicians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2019_9628_MOESM1_ESM.pptx
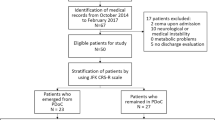
Supplementary figure 1: Study flowchart. CRS-R: Coma Recovery Scale-Revised; DRS: Disability Rating Scale; MCS: Minimally Conscious State; EMCS: Emergence from MCS; UWS: Unresponsive Wakefulness Syndrome (JPG 68 kb)
415_2019_9628_MOESM2_ESM.pptx
Supplementary figure 2: DRS at transition and at discharge for non-TBI and TBI. DRS total scores (means and SEs) at transition from UWS to MCS– (black columns) or at transition from UWS or MCS– to MCS+ (grey columns) and at discharge for the same groups. The graph on the left shows the results for the non-TBI subgroup and the graph on the right shows the results for the TBI subgroup (JPG 86 kb)
415_2019_9628_MOESM3_ESM.pptx
Supplementary figure 3: DRS without the communication subscale at time of transition. DRS total scores removing the communication subscale (means and SEs) for each group at transition from UWS to MCS– (black column) or at transition from UWS or MCS– to MCS+ (six grey columns). A3 = command following; C1 = intentional communication; DRS= Disability Rating Scale, MCS = minimally conscious state minus; O3 = intelligible verbalization. Black asterisks represent statistical differences between groups (JPG 88 kb)
Rights and permissions
About this article
Cite this article
Thibaut, A., Bodien, Y.G., Laureys, S. et al. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol 267, 1245–1254 (2020). https://doi.org/10.1007/s00415-019-09628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09628-y