Abstract
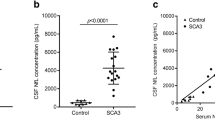
Blood biomarkers in degenerative ataxias are still largely missing. Here, we aimed to provide piloting proof-of-concept that serum Neurofilament light (NfL) could offer a promising peripheral blood biomarker in degenerative ataxias. Specifically, as a marker of neuronal damage, NfL might (1) help to differentiate multiple system atrophy of cerebellar type (MSA-C) from sporadic adult-onset ataxia (SAOA), and (2) show increases in repeat-expansion spinocerebellar ataxias (SCAs) which might be amenable to treatment in the future. To explore these two hypotheses, we measured serum NfL levels by single-molecule array (Simoa) technique in 115 subjects, comprising patients with MSA-C (n = 25), SAOA (n = 25), the most frequent repeat-expansion SCAs (SCA 1, 2, 3 and 6) (n = 20), and age-matched controls (n = 45). Compared to controls, NfL was significantly increased in MSA-C, with levels significantly higher than in SAOA (AUC = 0.74 (0.59–0.89), mean and 95% confidence interval, p = .004). NfL was also significantly increased in SCA patients as compared to controls (AUC = 0.91 (0.81–1.00), p < .001), including NfL increases in SCA1 and SCA3. These findings provide first proof-of-concept that NfL might provide a promising peripheral biomarker in degenerative ataxias, e.g. supporting the differentiation of MSA-C from SAOA, and indicating neuronal damage in repeat-expansion SCAs.

Similar content being viewed by others
References
Giordano I, Harmuth F, Jacobi H, Paap B, Vielhaber S, Machts J, Schols L, Synofzik M, Sturm M, Tallaksen C, Wedding IM, Boesch S, Eigentler A, van de Warrenburg B, van Gaalen J, Kamm C, Dudesek A, Kang JS, Timmann D, Silvestri G, Masciullo M, Klopstock T, Neuhofer C, Ganos C, Filla A, Bauer P, Tezenas du Montcel S, Klockgether T (2017) Clinical and genetic characteristics of sporadic adult-onset degenerative ataxia. Neurology 89:1043–1049
Scoles DR, Meera P, Schneider MD, Paul S, Dansithong W, Figueroa KP, Hung G, Rigo F, Bennett CF, Otis TS, Pulst SM (2017) Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 544:362–366
Keiser MS, Kordasiewicz HB, McBride JL (2016) Gene suppression strategies for dominantly inherited neurodegenerative diseases: lessons from Huntington’s disease and spinocerebellar ataxia. Hum Mol Genet 25:R53–R64
Paulson HL, Shakkottai VG, Clark HB, Orr HT (2017) Polyglutamine spinocerebellar ataxias—from genes to potential treatments. Nat Rev Neurosci 18:613–626
Toonen LJA, Rigo F, van Attikum H, van Roon-Mom WMC (2017) Antisense oligonucleotide-mediated removal of the polyglutamine repeat in spinocerebellar ataxia type 3 mice. Mol Ther Nucleic Acids 8:232–242
Wilke C, Preische O, Deuschle C, Roeben B, Apel A, Barro C, Maia L, Maetzler W, Kuhle J, Synofzik M (2016) Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. J Neurol Neurosurg Psychiatry 87:1270–1272
Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M (2016) Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91:56–66
Zetterberg H (2016) Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron 91:1–3
Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, Turner MR (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2:748–755
Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, Kilander L, Religa D, Wimo A, Winblad B, Rosengren L, Schott JM, Blennow K, Eriksdotter M, Zetterberg H (2014) CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83:1945–1953
Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, Liman V, Norgren N, Blennow K, Zetterberg H (2016) Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 54:1655–1661
Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J, Swiss Multiple Sclerosis Cohort Study G (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81:857–870
Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, Grobke S, Voortman M, Pirpamer L, Hofer E, Ropele S, Schmidt R, Kuhle J, Fazekas F, Khalil M (2017) Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89:2108–2114
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Abdo WF, van de Warrenburg BP, Munneke M, van Geel WJ, Bloem BR, Kremer HP, Verbeek MM (2006) CSF analysis differentiates multiple-system atrophy from idiopathic late-onset cerebellar ataxia. Neurology 67:474–479
Faul F, Erdfelder E, Buchner A, Lang A-G (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–1160
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Fancellu R (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720
Matsushima M, Yabe I, Oba K, Sakushima K, Mito Y, Takei A, Houzen H, Tsuzaka K, Yoshida K, Maruo Y, Sasaki H (2016) Comparison of different symptom assessment scales for multiple system atrophy. Cerebellum 15:190–200
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88:930–937
Sako W, Murakami N, Izumi Y, Kaji R (2015) Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis. J Neurol Sci 352:84–87
Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, Durr A, Marelli C, Globas C, Linnemann C, Schols L, Rakowicz M, Rola R, Zdzienicka E, Schmitz-Hubsch T, Fancellu R, Mariotti C, Tomasello C, Baliko L, Melegh B, Filla A, Rinaldi C, van de Warrenburg BP, Verstappen CC, Szymanski S, Berciano J, Infante J, Timmann D, Boesch S, Hering S, Depondt C, Pandolfo M, Kang JS, Ratzka S, Schulz J, Tezenas du Montcel S, Klockgether T (2011) The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology 77:1035–1041
Acknowledgements
Biosamples were obtained from the Neuro-Biobank of the University of Tübingen, Germany, which is supported by the University of Tübingen, the Hertie Institute for Clinical Brain Research (HIH) and the German Center for Neurodegenerative Diseases (DZNE). MS was supported by the Else Kröner-Fresenius-Stiftung.
Author information
Authors and Affiliations
Contributions
CW: design and conceptualisation of the study, acquisition of data, analysis of the data, drafting and revision of the manuscript. FB: acquisition of data, revision of the manuscript. SH: acquisition of data, revision of the manuscript. KB: acquisition of data, revision of the manuscript. LS: acquisition of data, revision of the manuscript. JK: acquisition and analysis of data, design and conceptualisation of measurements, revision of the manuscript. MS: design and conceptualisation of the study, acquisition of data, drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
JK’s institution (University Hospital Basel) received in the last 3 years and used exclusively for research support: consulting fees from Novartis, Protagen AG; speaker fees from the Swiss MS Society, Biogen, Novartis, Roche, Genzyme; travel expenses from Merck Serono, Novartis; grants from ECTRIMS Research Fellowship Programme, University of Basel, Swiss MS Society, Swiss National Research Foundation, Bayer (Switzerland) AG, Genzyme, Novartis. MS received speaker’s honoraria and research support from Actelion Pharmaceuticals, unrelated to the current project and manuscript. The other authors declare no competing financial interests.
Ethical statements
The university’s ethics committee approved the study; the methods were carried out in accordance with the relevant guidelines and regulations.
Informed consent
All the subjects gave a written informed consent prior to participation.
Data availability
The datasets analysed in the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilke, C., Bender, F., Hayer, S.N. et al. Serum neurofilament light is increased in multiple system atrophy of cerebellar type and in repeat-expansion spinocerebellar ataxias: a pilot study. J Neurol 265, 1618–1624 (2018). https://doi.org/10.1007/s00415-018-8893-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8893-9