Abstract
During the first meiotic prophase in male mammals, sex chromosomes undergo a program of transcriptional silencing called meiotic sex chromosome inactivation (MSCI). MSCI is triggered by accumulation of proteins like BRCA1, ATR, and γH2AX on unsynapsed chromosomes, followed by local changes on the sex chromatin, including histone modifications, incorporation of specific histone variants, non-histone proteins, and RNAs. It is generally thought that MSCI represents the transition of unsynapsed chromatin from a transcriptionally active state to a repressed state. However, transcription is generally low in the whole nucleus during the early stages of the first meiotic prophase, when markers of MSCI first appear, and is then reactivated globally during pachytene. Thus, an alternative possibility is that MSCI represents the targeted maintenance and/or reinforcement of a prior repressed state, i.e., a failure to reactivate. Here, we present an analysis of the temporal and spatial appearance of transcriptional and MSCI markers, as well as chromatin modifications related to transcriptional regulation. We show that levels of RNA pol II and histone H3 acetylated at lysine 9 (H3K9ac) are low during leptotene, zygotene, and early pachytene, but increase strongly in mid-pachytene, indicating that reactivation occurs with some delay after synapsis. However, while transcription markers appear abundantly on the autosomes at mid-pachytene, they are not directed to the sex chromosomes. Interestingly, we found that chromatin modifications related to transcriptional silencing and/or MSCI, namely, histone H3 trimethylated at lysine 9 (H3K9me3), histone H3 monomethylated at lysine 4 (H3K4me1), γH2AX, SUMO1, and XMR, appear on the sex chromosomes before autosomes become reactivated. These results suggest that the onset of MSCI during late zygotene and early pachytene may prevent sex chromosome reactivation during mid-pachytene instead of promoting inactivation de novo. Additionally, we found temporal differences between the X and Y chromosomes in the recruitment of DNA repair and MSCI markers, indicating a differential regulation of these processes. We propose that many of the meiotic defects attributed to failure to silence sex chromosomes could be interpreted as a more general process of transcriptional misregulation that occurs under certain pathological circumstances in zygotene and early pachytene.












Similar content being viewed by others
References
Almstrup K, Nielsen JE, Hansen MA, Tanaka M, Skakkebaek NE, Leffers H (2004) Analysis of cell-type-specific gene expression during mouse spermatogenesis. Biol Reprod 70:1751–1761
Ashley T (2002) X-autosome translocations, meiotic synapsis, chromosome evolution and speciation. Cytogenet Genome Res 96:33–39
Ashley T, Plug AW, Xu J et al (1995) Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104:19–28
Ashley T, Gaeth AP, Creemers LB, Hack AM, de Rooij DG (2004) Correlation of meiotic events in testis sections and microspreads of mouse spermatocytes relative to the mid-pachytene checkpoint. Chromosoma 113:126–136
Ayoub N, Richler C, Wahrman J (1997) Xist RNA is associated with the transcriptionally inactive XY body in mammalian male meiosis. Chromosoma 106:1–10
Baarends WM, Wassenaar E, van der Laan R et al (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25:1041–1053
Barchi M, Mahadevaiah S, Di Giacomo M et al (2005) Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol 25:7203–7215
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6:989–998
Baudat F, Buard J, Grey C et al (2010) PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327:836–840
Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD (2005) SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm-/- spermatocytes. J Cell Sci 118:3233–3245
Bellani MA, Boateng KA, McLeod D, Camerini-Otero RD (2010) The expression profile of the major mouse SPO11 isoforms indicates that SPO11beta introduces double strand breaks and suggests that SPO11alpha has an additional role in prophase in both spermatocytes and oocytes. Mol Cell Biol 30:4391–4403
Bolcun-Filas E, Bannister LA, Barash A et al (2011) A-MYB (MYBL1) transcription factor is a master regulator of male meiosis. Development 138:3319–3330
Borde V, Robine N, Lin W, Bonfils S, Geli V, Nicolas A (2009) Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J 28:99–111
Burgoyne PS, Mahadevaiah SK, Turner JM (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10:207–216
Chicheportiche A, Bernardino-Sgherri J, de Massy B, Dutrillaux B (2007) Characterization of Spo11-dependent and independent phospho-H2AX foci during meiotic prophase I in the male mouse. J Cell Sci 120:1733–1742
Cobb J, Cargile B, Handel MA (1999) Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol 205:49–64
Costa Y, Speed RM, Gautier P et al (2006) Mouse MAELSTROM: the link between meiotic silencing of unsynapsed chromatin and microRNA pathway? Hum Mol Genet 15:2324–2334
Cowell IG, Aucott R, Mahadevaiah SK et al (2002) Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma 111:22–36
Das NK, Siegel EP, Alfert M (1965) Synthetic activities during spermatogenesis in the locust. J Cell Biol 25:387–395
de Vries FA, de Boer E, van den Bosch M et al (2005) Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev 19:1376–1389
DeJong J (2006) Basic mechanisms for the control of germ cell gene expression. Gene 366:39–50
Eijpe M, Offenberg H, Goedecke W, Heyting C (2000) Localisation of RAD50 and MRE11 in spermatocyte nuclei of mouse and rat. Chromosoma 109:123–132
Escalier D, Garchon HJ (2000) XMR is associated with the asynapsed segments of sex chromosomes in the XY body of mouse primary spermatocytes. Chromosoma 109:259–265
Fernandez-Capetillo O, Mahadevaiah SK, Celeste A et al (2003) H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell 4:497–508
Godmann M, Auger V, Ferraroni-Aguiar V et al (2007) Dynamic regulation of histone H3 methylation at lysine 4 in mammalian spermatogenesis. Biol Reprod 77:754–764
Goetz P, Chandley AC, Speed RM (1984) Morphological and temporal sequence of meiotic prophase development at puberty in the male mouse. J Cell Sci 65:249–263
Handel MA (2004) The XY body: a specialized meiotic chromatin domain. Exp Cell Res 296:57–63
Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11:124–136
Henderson SA (1963) Differential ribonucleic acid synthesis of X and autosomes during meiosis. Nature 200:1235
Homolka D, Ivanek R, Capkova J, Jansa P, Forejt J (2007) Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res 17:1431–1437
Hoyer-Fender S (2003) Molecular aspects of XY body formation. Cytogenet Genome Res 103:245–255
Hoyer-Fender S, Costanzi C, Pehrson JR (2000) Histone macroH2A1.2 is concentrated in the XY-body by the early pachytene stage of spermatogenesis. Exp Cell Res 258:254–260
Hublitz P, Albert M, Peters AH (2009) Mechanisms of transcriptional repression by histone lysine methylation. Int J Dev Biol 53:335–354
Ichijima Y, Ichijima M, Lou Z et al (2011) MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev 25:959–971
Inagaki A, Schoenmakers S, Baarends WM (2010) DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics 5:255–266
Kauppi L, Barchi M, Baudat F, Romanienko PJ, Keeney S, Jasin M (2011) Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331:916–920
Khalil AM, Driscoll DJ (2006) Histone H3 lysine 4 dimethylation is enriched on the inactive sex chromosomes in male meiosis but absent on the inactive X in female somatic cells. Cytogenet Genome Res 112:11–15
Khil PP, Smirnova NA, Romanienko PJ, Camerini-Otero RD (2004) The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation. Nat Genet 36:642–646
Kierszenbaum AL, Tres LL (1974) Nucleolar and perichromosomal RNA synthesis during meiotic prophase in the mouse testis. J Cell Biol 60:39–53
Kimmins S, Sassone-Corsi P (2005) Chromatin remodelling and epigenetic features of germ cells. Nature 434:583–589
Kimmins S, Kotaja N, Davidson I, Sassone-Corsi P (2004) Testis-specific transcription mechanisms promoting male germ-cell differentiation. Reproduction 128:5–12
Kleene KC (2001) A possible meiotic function of the peculiar patterns of gene expression in mammalian spermatogenic cells. Mech Dev 106:3–23
Kota SK, Feil R (2010) Epigenetic transitions in germ cell development and meiosis. Dev Cell 19:675–686
Kralewski M, Benavente R (1997) XY body formation during rat spermatogenesis: an immunocytochemical study using antibodies against XY body-associated proteins. Chromosoma 106:304–307
Mahadevaiah SK, Turner JM, Baudat F et al (2001) Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 27:271–276
Mahadevaiah SK, Bourc'his D, de Rooij DG, Bestor TH, Turner JM, Burgoyne PS (2008) Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol 182:263–276
Manterola M, Page J, Vasco C et al (2009) A high incidence of meiotic silencing of unsynapsed chromatin is not associated with substantial pachytene loss in heterozygous male mice carrying multiple simple Robertsonian translocations. PLoS Genet 5:e1000625
Marcon E, Babak T, Chua G, Hughes T, Moens PB (2008) miRNA and piRNA localization in the male mammalian meiotic nucleus. Chromosome Res 16:243–260
Moens PB, Tarsounas M, Morita T et al (1999) The association of ATR protein with mouse meiotic chromosome cores. Chromosoma 108:95–102
Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B (2002) The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA–DNA interactions without reciprocal recombination. J Cell Sci 115:1611–1622
Monesi V (1964) Ribonucleic acid synthesis during mitosis and meiosis in the mouse testis. J Cell Biol 22:521–532
Monesi V (1965) Differential rate of ribonucleic acid synthesis in the autosomes and sex chromosomes during male meiosis in the mouse. Chromosoma 17:11–21
Moses MJ (1980) New cytogenetic studies on mammalian meiosis. In: Serio M, Martini L (eds) Animal models in human reproduction. Raven Press, New York, pp 169–190
Ortega S, Prieto I, Odajima J et al (2003) Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 35:25–31
Page J, Berrios S, Rufas JS et al (2003) The pairing of X and Y chromosomes during meiotic prophase in the marsupial species Thylamys elegans is maintained by a dense plate developed from their axial elements. J Cell Sci 116:551–560
Page J, de la Fuente R, Gomez R et al (2006) Sex chromosomes, synapsis, and cohesins: a complex affair. Chromosoma 115:250–259
Pang ALY, Johnson W, Ravindranath N, Dym M, Rennert OM, Chan W-Y (2006) Expression profiling of purified male germ cells: stage-specific expression patterns related to meiosis and postmeiotic development. Physiol Genomics 24:75–85
Peters AH, Plug AW, van Vugt MJ, de Boer P (1997) A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res 5:66–68
Peters AH, O'Carroll D, Scherthan H et al (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107:323–337
Plug AW, Peters AH, Keegan KS, Hoekstra MF, de Boer P, Ashley T (1998) Changes in protein composition of meiotic nodules during mammalian meiosis. J Cell Sci 111(Pt 4):413–423
Reinholdt LG, Czechanski A, Kamdar S, King BL, Sun F, Handel MA (2009) Meiotic behavior of aneuploid chromatin in mouse models of Down syndrome. Chromosoma 118:723–736
Reynard LN, Turner JM, Cocquet J et al (2007) Expression analysis of the mouse multi-copy X-linked gene Xlr-related, meiosis-regulated (Xmr), reveals that Xmr encodes a spermatid-expressed cytoplasmic protein, SLX/XMR. Biol Reprod 77:329–335
Rogers RS, Inselman A, Handel MA, Matunis MJ (2004) SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma 113:233–243
Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6:975–987
Royo H, Polikiewicz G, Mahadevaiah SK et al (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20:2117–2123
Schimenti J (2005) Synapsis or silence. Nat Genet 37:11–13
Schultz N, Hamra FK, Garbers DL (2003) A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA 100:12201–12206
Shima JE, McLean DJ, McCarrey JR, Griswold MD (2004) The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod 71:319–330
Solari AJ (1969) The evolution of the ultrastructure of the sex chromosomes (sex vesicle) during meiotic prophase in mouse spermatocytes. J Ultrastruct Res 27:289–305
Solari AJ (1974) The behavior of the XY pair in mammals. Int Rev Cytol 38:273–317
Solari AJ (1993) Sex chromosomes and sex determination in vertebrates. CRC Press, Boca Raton
Tarsounas M, Morita T, Pearlman RE, Moens PB (1999) RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol 147:207–220
Tres LL (1977) Extensive pairing of the XY bivalent in mouse spermatocytes as visualized by whole-mount electron microscopy. J Cell Sci 25:1–15
Turner JM (2007) Meiotic sex chromosome inactivation. Development 134:1823–1831
Turner JM, Mahadevaiah SK, Benavente R, Offenberg HH, Heyting C, Burgoyne PS (2000) Analysis of male meiotic “sex body” proteins during XY female meiosis provides new insights into their functions. Chromosoma 109:426–432
Turner JM, Aprelikova O, Xu X et al (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14:2135–2142
Turner JM, Mahadevaiah SK, Fernandez-Capetillo O et al (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37:41–47
van der Heijden GW, Derijck AA, Posfai E et al (2007) Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet 39:251–258
van Dijk K, Marley KE, B-r J et al (2005) Monomethyl histone H3 lysine 4 as an epigenetic mark for silenced euchromatin in Chlamydomonas. Plant Cell Online 17:2439–2453
Viera A, Calvente A, Page J et al (2004) X and B chromosomes display similar meiotic characteristics in male grasshoppers. Cytogenet Genome Res 106:302–308
Viera A, Rufas JS, Martinez I, Barbero JL, Ortega S, Suja JA (2009) CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. J Cell Sci 122:2149–2159
Vigodner M (2009) Sumoylation precedes accumulation of phosphorylated H2AX on sex chromosomes during their meiotic inactivation. Chromosome Res 17:37–45
Wang PJ, McCarrey JR, Yang F, Page DC (2001) An abundance of X-linked genes expressed in spermatogonia. Nat Genet 27:422–426
Wang PJ, Page DC, McCarrey JR (2005) Differential expression of sex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum Mol Genet 14:2911–2918
Acknowledgements
We express our sincere thanks to Denise Escalier (Université Paris 5, Paris, France) for providing the XMR antibody and to Dr. Teresa L. Mastracci and Dr. Juan Luis Santos (Universidad Complutense de Madrid) for their critical review of the manuscript. This work was supported by grant BFU2009-10987 from Ministerio de Ciencia e Innovación, grants A/017762/08 and A/023249/09 from Agencia Española de Cooperación Internacional para el Desarrollo (Spain), and FONDECYT grant 1080090 (Chile).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dr. Scott Keeney
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Figure 1
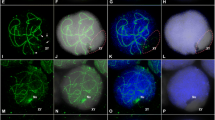
Location of SCP3 (green), centromeres (red) and γH2AX (blue) during late zygotene. (A). Most autosomes are still undergoing synapsis. γH2AX is present in both synapsed and unsynapsed autosomes. The centromeres are located in distal position in all autosomes. The X chromosome (enlarged in B) also presents a centromere located at one end of its AE (arrowhead). The opposite end has a thickening corresponding to the PAR (arrow). This chromosome is completely labeled with γH2AX, except in the pericentromeric region. The Y chromosome (enlarged in C) presents its centromere located in a sub-distal position (arrowhead), thus showing a visible short arm. This chromosome shows a thickening of the AE at the opposite end (arrow), corresponding with the PAR. γH2AX labeling is restricted to one chromosome end, presumably stemming from the transition between the thickened and the regular region of its AE. (JPEG 31 kb)
Supplementary Figure 2
DAPI staining of sex chromosomes in the same cells shown in Figure 1. A distinct conformation of the sex chromatin is not detected during zygotene (A–B) or early pachytene (C–D). A different conformation is only observed from mid pachytene onwards (E–L). rb indicates the round body, which is usually associated with the sex chromosomes from mid pachytene onwards. (JPEG 64 kb)
Supplementary Figure 3
Location of SCP3 (green) and γH2AX (red) during mid and late prophase-I. (A–C). Early-mid pachytene. All the autosomes are completely synapsed and sex chromosomes also show a great extension of synapsis. When exposure of the picture is adjusted to the intensity of the labeling on the sex chromosomes (A) γH2AX is not detectable in the chromatin of autosomes. However, if the picture is over exposed (B–C), an intense labeling emerges on the chromatin of all autosomal bivalents. This is mostly restricted to the chromatin around the LEs (arrowheads) and clearly does not involve the LEs (see enlarged detail in the upper insert). Additionally, some bivalents show great γH2AX signals that emanate from the LEs and seem to expand to greater chromatin loops (arrow). (D–F). Mid pachytene. γH2AX labeling on the sex chromosomes now depicts a more regular border of the sex body. Upon over-exposure of the pictures (E–F) the labeling on the autosomes is still visible in the chromatin close to the LEs, but γH2AX is now concentrated in some specific regions along bivalents, yielding a banded-like pattern (arrows). (G–I). Late pachytene. The sex body remains intensely labeled with γH2AX. The labeling on the autosomes in now restricted to a few spots (arrows) located over some but not all bivalents. (J–L). Diplotene. γH2AX labeling of the sex body is still intense. No γH2AX in detected on the autosomes at this stage. (JPEG 103 kb)
Rights and permissions
About this article
Cite this article
Page, J., de la Fuente, R., Manterola, M. et al. Inactivation or non-reactivation: what accounts better for the silence of sex chromosomes during mammalian male meiosis?. Chromosoma 121, 307–326 (2012). https://doi.org/10.1007/s00412-012-0364-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-012-0364-y