Abstract
Objective
β2-Adrenoceptor agonists are widely used to treat asthma because of their bronchial-dilation effects. We previously reported that isoprenaline, via the apical and basolateral β2-adrenoceptor, induced Cl− secretion by activating cyclic AMP (cAMP)-dependent pathways in human bronchial epithelia. Despite these results, whether and how the β2-adrenoceptor-mediated cAMP-dependent pathway contributes to pro-inflammatory cytokine release in human bronchial epithelia remains poorly understood.
Methods
We investigated β2-adrenoceptor-mediated signaling pathways involved in the production of two pro-inflammatory cytokines, interleukin (IL)-6 and IL-8, in 16HBE14o- human bronchial epithelia. The effects of isoprenaline or formoterol were assessed in the presence of protein kinase A (PKA), exchange protein directly activated by cAMP (EPAC), Src, and extracellular signal-regulated protein kinase (ERK)1/2 inhibitors. The involvement of β-arrestin2 was examined using siRNA knockdown.
Results
Isoprenaline and formoterol (both β2 agonists) induced IL-6, but not IL-8, release, which could be inhibited by ICI 118,551 (β2 antagonist). The PKA-specific inhibitor, H89, partially inhibited IL-6 release. Another intracellular cAMP receptor, EPAC, was not involved in IL-6 release. Isoprenaline-mediated IL-6 secretion was attenuated by dasatinib, a Src inhibitor, and PD98059, an ERK1/2 inhibitor. Isoprenaline treatment also led to ERK1/2 phosphorylation. In addition, knockdown of β-arrestin2 by siRNA specifically suppressed cytokine release when a high concentration of isoprenaline (1 mM) was used.
Conclusion
Our results suggest that activation of the β2-adrenoceptor in 16HBE14o- cells stimulated the PKA/Src/ERK1/2 and/or β-arrestin2 signaling pathways, leading to IL-6 release. Therefore, our data reveal that β2-adrenoceptor signaling plays a role in the immune regulation of human airway epithelia.
Similar content being viewed by others
Introduction
β2-Adrenoceptor agonists are the most widely used and effective therapies for ameliorating asthmatic symptoms. However, clinical application of β2-adrenoceptor agonists has been shown to enhance airway inflammation [1], triggering a paradox in asthma management [2, 3]. During the inhalation of β2-adrenoceptor agonists, airway epithelial cells are exposed to the drug, leading to potential side effects that may also require attention. It has been reported that β2-adrenoceptor activation in the airway epithelium can restore and promote the cardinal features of asthma, including eosinophilic inflammation, mucous metaplasia, and airway hyperresponsiveness [4]. Therefore, although β2-adrenoceptor agonists are expected to dilate airway smooth muscle, their activation of epithelial β2-adrenoceptors may play a major role in causing inflammation in patients taking these therapeutics, which could in turn lead to further asthma symptoms.
β2-Adrenoceptor agonists are known to activate cyclic AMP (cAMP)-dependent kinase signaling pathways [5, 6]. We recently reported that isoprenaline stimulated β2-adrenoceptors and induced Cl− secretion, which was mediated through the cAMP-dependent protein kinase A (PKA) pathway, leading to the activation of cystic fibrosis transmembrane conductance regulator (CFTR) in human bronchial epithelia [7]. However, whether the cAMP/PKA signaling pathway is involved in aggravating inflammation by promoting secretion of pro-inflammatory cytokines remains largely unknown. Apart from PKA, exchange protein directly activated by cAMP (EPAC) has been found to be a novel cAMP-responding downstream molecule associated with many biological functions [8].
Moreover, studies have shown that β2-adrenoceptor agonists activate G protein-independent pathways mediated by β-arrestins [9], which were initially expected to mediate receptor internalization and desensitization [10]. The latest findings indicate that β-arrestins, acting as scaffolds to recruit signaling molecules, are capable of activating Src family non-receptor tyrosine kinases and mitogen-activated protein kinases (MAPKs), which then proceed to activate pro-inflammatory cytokine release [11, 12].
In this study, we examined the involvement of both PKA and EPAC in β2-adrenoceptor activation to expand our knowledge of cAMP-dependent pathways in asthma. The effects of common pro-inflammatory pathways, such as Src and extracellular signal-regulated protein kinase (ERK)1/2, were also examined in our study. We characterized the release of interleukin (IL)-6 and IL-8 induced by various concentrations of a β2-adrenoceptor agonist, isoprenaline, and a more specific β2-adrenoceptor agonist, formoterol. Our data demonstrate that β2-adrenoceptor activation led to IL-6, but not IL-8, production. PKA/Src, as well as ERK1/2, activated IL-6 release when relatively low concentrations of isoprenaline were used. However, β-arrestin2 was involved in IL-6 expression following treatment with high concentrations of isoprenaline. These findings expand our knowledge of the important role β2-adrenoceptor agonists play in human airway inflammation and thus have clinical implications for future asthma research and therapy.
Materials and Methods
Reagents
All pharmacological tools were obtained from Sigma-Aldrich (St Louis, MO, USA). All cell culture reagents were obtained from Invitrogen (Carlsbad, CA, USA), unless otherwise stated.
Cell Culture
The human 16HBE14o- bronchial epithelia cell line was cultured and maintained as described previously [13]. After cells reached confluence, they were placed in serum-free conditions overnight before drug delivery and sample collection. For experiments, cells were grown in 24-well culture plates [14].
ELISA
Cells were grown in 24-well culture plates and cell-free supernatants were collected from control and treated cells before analysis using a commercially available ELISA kit specific for IL-6 (Invitrogen eBioscience, Carlsbad, CA, USA) and IL-8 (BD Biosciences, San Diego, CA, USA) according to the manufacturers’ protocols. All experiments were performed in duplicate.
Small Interference RNA Transfection
β-arrestin2 knockdown by small interference RNA (siRNA) was performed as described previously [14]. Briefly, the medium was changed to OPTI-MEM™ I Reduced Serum Medium (Gibco, Invitrogen, USA) after cells reached 60%–70% confluence. Transfection of 25 nM β-arrestin2 siRNA (Thermo Fisher Scientific, Waltham, MA, USA, Cat.: AM16708) or control siRNA (Thermo Fisher Scientific, Cat.: AM4613) was achieved using Lipofectamine® RNAiMAX Reagent (Invitrogen) 24 h before isoprenaline was added.
Western Blotting
SDS-PAGE (Bio-Rad Laboratories, Hercules, CA, USA) followed by transfer to polyvinylidene fluoride membranes (Immobilon-P, Millipore Corporation, Billerica, MA, USA) was performed. Anti-phospho-p44/42 MAPK (ERK 1/2; Cell Signaling Technology; Danvers, MA; 1:1,000) and anti-p44/42 MAPK (ERK 1/2; Cell Signaling Technology; 1:1,000) primary antibodies were used.
Real-Time PCR
Total RNA was extracted using TRIzol™ Reagent and reverse transcribed into cDNA using iScript™ Reverse Transcription Supermix (Bio-Rad Laboratories). Real-time PCR was conducted using the Applied Biosystems Power SYBR Green PCR Master Mix in an ABI QuantStudio1 thermal cycler (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). The following primer sequences were used (5’-3’): gapdh forward, CGGGAAGGAAATGAATGGGC; reverse, GCCCAATACGACCAAATCAGAGAAT; beta-arrestin2 forward, GGAAGCTGGGCCAGCAT; reverse, TGTGACGGAGCATGGAAGATT.
Statistical Analysis
Data are expressed as the means ± standard error (S.E.). Comparisons between control and treated epithelia were performed using the Student’s t test or one-way analysis of variance (ANOVA) with Dunnett’s post hoc test (GraphPad Prism 5) as appropriate.
Results
β 2-Adrenoceptor is Involved in Isoprenaline-Induced Secretion of IL-6 But Not IL-8
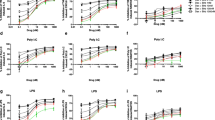
We previously demonstrated that isoprenaline activates β2-adrenoceptor in 16HBE14o- cells to evoke Cl− secretion via a cAMP-dependent pathway [7]. Here, we examined the involvement of β2-adrenoceptors using pharmacological tools and quantified isoprenaline-induced IL-6 and IL-8 production by ELISA. Stimulation of 16HBE14o- cells with different concentrations of isoprenaline (0.1 nM – 1 μM) for 6 h caused an increase in IL-6 secretion in a concentration-dependent manner (Fig. 1A). Compared to IL-6 secretion, the same concentrations of isoprenaline did not cause a significant increase in IL-8, except at the highest concentration of 1 μM (Fig. 1B). The specific α1-receptor antagonist prazosin and the β-receptor antagonist propranolol were employed to verify the involvement of α-and β-adrenoceptors in IL-6 secretion (Fig. 1C). Cells were pretreated with prazosin (1 μM) or propranolol (10 μM) for 2 h and then stimulated with isoprenaline in the presence of the antagonists for 6 h. Figure 1C shows that propranolol (10 μM) but not prazosin (1 μM) inhibited isoprenaline-induced IL-6 release when compared to the respective controls without inhibitor. To further demonstrate the involvement of the β2-adrenoceptor, we examined the effect of formoterol, a specific β2-adrenoceptor agonist, and ICI 118,551, a specific β2-adrenoceptor antagonist, on IL-6 and IL-8 secretion. Similar to the effects of isoprenaline, formoterol-induced IL-6 (Fig. 1D) but not IL-8 (Fig. 1E) release in a concentration-dependent manner. The effects of both isoprenaline and formoterol on IL-6 production were significantly blocked by ICI 118,551 (Fig. 1F). Taken together, these results confirmed that isoprenaline could induce IL-6 secretion by activating β2-adrenoceptor. Because isoprenaline and formoterol did not significantly increase IL-8 secretion, the inhibitory effects of prazosin, propranolol, and ICI 118,551 on isoprenaline- or formoterol-induced cytokine secretion were not investigated further.
Isoprenaline or formoterol induce IL-6 and IL-8 release. A–B 16HBE14o- cells were treated with different concentrations of isoprenaline for 6 h. IL-6 (A) and IL-8 (B) release were quantified by ELISA (n = 5–7). C Cells were pretreated with prazosin (1 μM) or propranolol (10 μM) for 2 h, and then the cells were stimulated with isoprenaline in the presence of the inhibitors prior to quantification of IL-6 secretion by ELISA (n = 4–6). D–E 16HBE14o- cells were treated with different concentrations of formoterol for 6 h and then IL-6 (D) and IL-8 (E) release were quantified by ELISA (n = 4–5). F Cells were pretreated with ICI 118,551 (10 μM; ICI) for 2 h before stimulation of the cells with isoprenaline or formoterol for 6 h (n = 3–5). Each column represents the mean ± S.E. (For A, B, D & E: *p < 0.05 compared with the control (ctl) group; one-way ANOVA with Dunnett’s post hoc test; For C & F: *p < 0.05 compared with the same concentration of isoprenaline or formoterol without inhibitor; Student's t-test)
cAMP-Dependent Signaling Pathways Mediate Isoprenaline-Induced IL-6 Release
We previously showed that isoprenaline could activate β2-adrenoceptor-dependent signaling pathways in 16HBE14o- cells, leading to an increase in intracellular cAMP level [7]. In this study, we investigated the involvement of two intracellular receptors of cAMP, namely PKA and EPAC, on IL-6 secretion. The effect of EPAC activation was examined by using an analog of cAMP (8-pCPT-2’-O-Me-cAMP; 8-CPT) which specifically activates EPAC protein [15]. We also used ESI-09, a novel non-cyclic nucleotide EPAC antagonist [16]. Compared to control samples, pretreatment of the epithelial cells with 5 µM 8-CPT or ESI-09 for 2 h before stimulating the cells with different concentrations of isoprenaline did not affect IL-6 secretion (Fig. 2A), suggesting that EPAC protein is not involved in isoprenaline-induced IL-6 secretion. However, treatment with 10 μM H89, a PKA-specific inhibitor, significantly suppressed isoprenaline-induced IL-6 release (Fig. 2B). Activation of adenylyl cyclase (AC) by forskolin has been reported to elevate intracellular cAMP level in 16HBE14o- cells [17], presumably leading to activation of PKA and EPAC. Stimulating the cells with 1000 nM isoprenaline for 6 h increased the IL-6 secretion (1313.7 ± 137.4 pg/ml; n = 10). This increase was not statistically different from treatment with isoprenaline and 5 µM forskolin (1130.0 ± 449.1 pg/ml; n = 4; p > 0.05 vs isoprenaline only; Student’s t test) or isoprenaline together with 5 µM forskolin and ESI-09 (1465.4 ± 160.1 pg/ml; n = 6; p > 0.05 vs isoprenaline only; Student’s t test). Therefore, no synergistic increase in IL-6 secretion was observed when epithelial cells were treated with both a β2-adrenoceptor agonist and cAMP-mobilizing agent. Similar results were obtained when the cells were treated with 10 nM isoprenaline (data not shown). Taken together, these results indicate that the IL-6 secretion stimulated by β2-adrenoceptor activation utilizes the same signaling pathway induced by forskolin treatment, namely the AC/cAMP signaling cascade followed by PKA but not EPAC activation.
cAMP-dependent signaling pathways are involved in isoprenaline-mediated IL-6 release. A Cells were pretreated with EPAC activator 8-CPT (5 μM) or EPAC inhibitor ESI-09 (5 μM) for 2 h before addition of isoprenaline (iso) for 6 h. IL-6 was quantified by ELISA. B The effect of PKA inhibitor H89 (10 μM) on isoprenaline-induced IL-6 was examined. Each column represents the mean ± S.E. n = 3–5; *p < 0.05, as calculated by the Student's t-test
Involvement of Src and ERK1/2 Signaling Pathways in Isoprenaline-Induced IL-6 Production
A recent study suggested that Src-mediated MAPK signaling pathways are involved in kidney inflammation [18]. To delineate whether Src, a typical family of non-receptor tyrosine kinases, is also involved in isoprenaline-induced IL-6 secretion, 16HBE14o- cells were stimulated with different concentrations of isoprenaline in the presence or absence of dasatinib, a Src inhibitor [19]. Figure 3A shows that treatment with 10 μM dasatinib significantly suppressed 10 nM isoprenaline-induced IL-6 secretion.
Src and ERK1/2 are involved in isoprenaline-induced IL-6 secretion. A–B Cells were treated with dasatinib (10 μM) or PD98059 (10 μM) for 2 h before isoprenaline (iso) treatment at different concentrations for 6 h. The effect of dasatinib (A) and PD98059 (B) on IL-6 release was examined. Each column represents the mean ± S.E. n = 4–6; *p < 0.05, as calculated by the Student's t-test
ERK1/2 is an important pro-inflammatory cytokine release mediator among different MAPKs in human airway epithelial cells [20]. Treatment of the cells with an ERK1/2 inhibitor, PD98059 (10 μM), reduced the level of IL-6 release induced by isoprenaline (Fig. 3B). Examination of ERK1/2 phosphorylation status by western blot analysis revealed that different concentrations of isoprenaline can induce ERK1/2 phosphorylation (Fig. 4A, C). Moreover, PD98059 significantly inhibited basal (DMSO) and isoprenaline-induced ERK1/2 phosphorylation (Fig. 4B, D). These data suggest that the Src and ERK1/2 signaling pathways are involved in isoprenaline-induced IL-6 secretion.
Effect of isoprenaline on ERK1/2 phosphorylation. A 16HBE14o- cells were stimulated with different concentrations of isoprenaline for 5 min. B Cells were treated with DMSO or PD98059 (10 μM) for 15 min followed by isoprenaline (10 μM) stimulation for 5 min. Representative images of western blots are shown. n = 3. Summarized data are shown in C and D, showing the quantification of p-ERK levels normalized to total ERK (*p < 0.05, as calculated by the Student's t-test or ANOVA as appropriate)
β-Arrestin2 Plays a Role in Isoprenaline-Induced IL-6 Production
β-arrestin2 is involved in Src and MAPK signaling pathways and inflammatory responses [12, 21]. To examine the involvement of β-arrestin2 in isoprenaline-induced IL-6 secretion, we depleted β-arrestin2 mRNA by siRNA (Fig. 5A) and studied the effect of this knockdown on IL-6 production. β-arrestin2 knockdown led to a significant decrease in IL-6 secretion induced by a relatively high concentration of isoprenaline (1 μM) (Fig. 5B).
β-Arrestin2 mediates isoprenaline-induced IL-6 release. A The efficiency of β-arrestin2 knockdown (KD) was verified by real-time PCR (n = 3). The expression of β-arrestin2 mRNA was normalized by the level of GAPDH mRNA. B The effect of β-arrestin2 KD on isoprenaline-induced IL-6 release was examined. Each data point represents the mean ± S.E. n = 5; *p < 0.05 compared with the same concentration of isoprenaline between the control (ctl siRNA) and KD groups (β-arrestin2 siRNA), as calculated by the Student’s t-test
Discussion
In the present study, we found that isoprenaline-induced IL-6, but not IL-8, production via β2-adrenoceptor-mediated signaling pathways. After activation of AC/cAMP signaling, PKA, but not EPAC, played a role in isoprenaline-mediated IL-6 secretion. In addition, both Src- and ERK1/2-dependent signaling pathways were involved. β-Arrestin2, however, was only involved in mediating isoprenaline-induced IL-6 production at relatively high concentrations. Thus, these results indicate that β2-adrenoceptor/AC/cAMP/PKA, Src and ERK1/2 are involved in mediating IL-6 release in 16HBE14o- cells. IL-6 and IL-8 are the two pro-inflammatory cytokine markers secreted by human airway epithelia during inflammation [20, 22]. These two classic proinflammatory cytokines are implicated in the initiation and perpetuation of local airway inflammatory responses and play important roles in bronchial epithelial cells [23, 24]. Our data suggest that activation of β2-adrenoceptor is only associated with IL-6, but not IL-8, secretion. IL-6 is elevated in asthmatic patients [25] and it is suggested that IL-6 has a causative role in determining an increase in airway resistance in asthma and chronic obstructive pulmonary disease [26].
PKA and EPAC are the two major downstream targets of cAMP [8]. Since β2-adrenoceptors are known to activate the AC/cAMP-dependent pathway in 16HBE14o- cells to activate CFTR, we examined the role of PKA and EPAC in isoprenaline-induced cytokine release. EPAC has been reported to take part in mediating inflammation [27] and activating ERK1/2 [28]. PKA and EPAC either cooperate [29, 30] or act independently [31] to regulate many biological functions. Although EPAC protein is expressed in 16HBE14o- cells [32], the results in Fig. 2 suggest that PKA, but not EPAC, participates in isoprenaline-induced IL-6 release. Our results are in contrast with other reports which suggest that the β2-adrenoceptor is associated with PKA-independent factors, such as EPAC. β2-adrenoceptor agonist induced IL-1β and IL-6 production through PKA-independent mechanisms in a murine macrophage cell line [33]. Activation of β2-adrenoceptors induced IL-6 production in neonatal mouse cardiac fibroblasts through an AC/cAMP/p38 MAPK pathway that was independent of PKA [34]. One possibility is that the involvement of EPAC in cytokine release appears to be cell-type-specific and may be related to pathophysiology during inflammation and specific innate immune protection in different tissues. Another possibility is that the aforementioned studies employed such high concentrations of β2-adrenoceptor agonist (e.g., 0.5 μM salmeterol; 10 μM isoprenaline) that cAMP-independent pathways such as β-arrestin2 become activated (Fig. 5). To the best of our knowledge, we are the first to study the effects of β2-adrenoceptor agonists across a wide range of concentrations, including relatively low concentrations (from 0.1 nM to 1 μM), on cytokine release.
Similar to PKA (Fig. 2B), the Src family tyrosine kinase inhibited IL-6 secretion (Fig. 3). Dasatinib is an FDA-approved small-molecule drug used to treat myeloid leukemia [35] that potently inhibits Src family kinase [36]. PKA is reported to be able to activate Src signaling by phosphorylating the kinase at residue serine 17 [37, 38]. Whether PKA suppresses IL-6 secretion through Src remains to be studied. It has been widely reported that β2-adrenoceptor agonist-mediated cytokine release occurs through ERK1/2 and/or p38 MAPK [9, 33, 34]. Our study is consistent with these reports as we demonstrate that PD98059 not only inhibited ERK1/2 phosphorylation (Fig. 4) but also suppressed IL-6 secretion (Fig. 3B).
β-Arrestin2 knockdown specifically inhibited the effect of high isoprenaline concentrations (Fig. 5). G protein-coupled receptor kinases (GRKs) typically mediate β-adrenoceptor phosphorylation followed by β-arrestin recruitment. β-arrestins act as a scaffold to further activate other signaling molecules, such as ERK1/2 [12, 39]. Our results strongly suggest that β-arrestin2 mediates β2-adrenoceptor agonist (e.g., isoprenaline)-induced cytokine release. This may be the case when patients are exposed to a relatively high concentration of agonists. Apart from IL-6, we observed an inhibitory effect of β-arrestin2 knockdown on IL-8 secretion induced by 1 µM isoprenaline (data not shown). Therefore, it is possible that GRK may phosphorylate the β2-adrenoceptor upon stimulation and induce β-arrestin2 recruitment to inhibit cytokine release via ERK1/2. The cAMP pathway has been shown to activate either p38 MAPK [40] or ERK1/2 [41]. Src can also activate ERK1/2 [42] and could be activated by PKA [38]. Whether Src and ERK1/2 signaling pathways are downstream of PKA has yet to be determined and awaits further investigation. Delineating the relationships between these signals would provide additional insights into the molecular events induced by β2-adrenoceptor activation.
Evidence that β2-adrenoceptor agonists are a double-edged sword in terms of asthma treatment is accumulating. Activation of cAMP/PKA pathways leads to the beneficial effects of bronchial dilation, while the involvement of β-arrestin pathways may lead to undesirable side effects, such as inflammation aggravation [11]. Bronchodilators, such as β2 agonists, are essential medicines used to manage symptomatic asthma. To induce bronchodilation, repeated use or increasing doses of β2-adrenoceptor agonist inevitably cause the airway epithelial cells to be exposed to the drug. In fact, the airway epithelial cells are the first physiochemical barrier to encounter the β2 agonist. Our results show how the use of β2-adrenoceptor drugs might affect the airway epithelia by releasing the pro-inflammatory cytokine IL-6. Further studies are required to identify other possible pro-inflammatory cytokines that are secreted upon β2 adrenoceptor activation in human airway epithelia.
Conclusion
The present study demonstrates that treatment with isoprenaline (0.1 nM–1 μM) induces IL-6, but not IL-8, secretion via the β2-adrenoceptor in human bronchial epithelia. Several signaling pathways, including PKA, Src, and ERK1/2, are involved. High concentrations of isoprenaline (1 μM) also activate the β-arrestin2 pathway. Our findings enhance our understanding of β2-adrenoceptor-mediated signaling pathways and their relationship with IL-6 release in human bronchial epithelia. While activation of the β2-adrenoceptor in smooth muscle cells could have a bronchodilatory therapeutic effect, this is counteracted by secretion of the pro-inflammatory cytokine IL-6 by the bronchial epithelium. Thus, the paradoxical effects of β2-adrenoceptor activation could hamper wide spread clinical use of β2-adrenoceptor agonists in treating asthma.
Abbreviations
- AC:
-
Adenylyl cyclase
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- AMP:
-
Adenosine monophosphate
- 8-CPT-cAMP:
-
8-(4-Chlorophenylthio)adenosine-3′,5′-cyclic Monophosphorothioate
- cAMP:
-
Cyclic AMP
- EPAC:
-
Exchange protein directly activated by cAMP
- ERK1/2:
-
Extracellular signal‑regulated protein kinase
- GRK:
-
G protein-coupled receptor kinase
- hrs:
-
Hours
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- MAPK:
-
Mitogen-activated protein kinase
- PKA:
-
Protein kinase A
References
Knight JM, Mak G, Shaw J et al (2015) Long-acting beta agonists enhance allergic airway disease. PLoS ONE 10:e142212
Martin MJ, Harrison TW (2019) Is it time to move away from short-acting beta-agonists in asthma management? Eur Respir J 53:1802223
Beasley R, Bird G, Harper J, Weatherall M (2018) The further paradoxes of asthma management: time for a new approach across the spectrum of asthma severity. Eur Respir J 52:1800694
Nguyen LP, Al-Sawalha NA, Parra S et al (2017) b2-Adrenoceptor signaling in airway epithelial cells promotes eosinophilic inflammation, mucous metaplasia, and airway contractility. Proc Natl Acad Sci USA 114:E9163–E9171
Anderson GP (2006) Current issues with beta2-adrenoceptor agonists: pharmacology and molecular and cellular mechanisms. Clin Rev Allergy Immunol 31:119–130
Johnson M (1998) The beta-adrenoceptor. Am J Respir Crit Care Med 158:S146-153
Zhang RG, Yip CY, Pan KW, Cai MY, Ko WH (2020) β2 adrenoceptor signaling regulates ion transport in 16HBE14o- human airway epithelial cells. J Cell Physiol 235:8387–8401
Robichaux WG 3rd, Cheng X (2018) Intracellular cAMP Sensor EPAC: physiology, pathophysiology, and therapeutics development. Physiol Rev 98:919–1053
Shenoy SK, Drake MT, Nelson CD et al (2006) beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281:1261–1273
Vaughan DJ, Millman EE, Godines V et al (2006) Role of the G protein-coupled receptor kinase site serine cluster in beta2-adrenergic receptor internalization, desensitization, and beta-arrestin translocation. J Biol Chem 281:7684–7692
Walker JK, DeFea KA (2014) Role for β-arrestin in mediating paradoxical β2AR and PAR2 signaling in asthma. Curr Opin Pharmacol 16:142–147
Peterson YK, Luttrell LM (2017) The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev 69:256–297
Zhang RG, Pan K, Hao Y, Yip CY, Ko WH (2019) Anti-inflammatory action of HO-1/CO in human bronchial epithelium in response to cationic polypeptide challenge. Mol Immunol 105:205–212
Zhang RG, Yip CY, Ko WH (2018) Carbon monoxide inhibits cytokine and chloride secretion in human bronchial epithelia. Cell Physiol Biochem 49:626–637
Henquin JC, Nenquin M (2014) Activators of PKA and Epac distinctly influence insulin secretion and cytosolic Ca2+ in female mouse islets stimulated by glucose and tolbutamide. Endocrinology 155:3274–3287
Wang X, Luo C, Cheng X, Lu M (2017) Lithium and an EPAC-specific inhibitor ESI-09 synergistically suppress pancreatic cancer cell proliferation and survival. Acta Biochim Biophys Sin (Shanghai) 49:573–580
Hao Y, Chow AW, Yip WC et al (2016) G protein-coupled estrogen receptor inhibits the P2Y receptor-mediated Ca2+ signaling pathway in human airway epithelia. Pflug Arch 468:1489–1503
Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P (2020) Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother 122:109772
Lindauer M, Hochhaus A (2018) Dasatinib. Recent Results Cancer Res 212:29–68
Hao Y, Liang JF, Chow AW, Cheung WT, Ko WH (2014) P2Y6 receptor-mediated proinflammatory signaling in human bronchial epithelia. PLoS ONE 9:e106235
Nichols HL, Saffeddine M, Theriot BS et al (2012) β-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci USA 109:16660–16665
Chow AW, Liang JF, Wong JS, Fu Y, Tang NL, Ko WH (2010) Polarized secretion of interleukin (IL)-6 and IL-8 by human airway epithelia 16HBE14o- cells in response to cationic polypeptide challenge. PLoS ONE 5:e12091
Asokananthan N, Graham PT, Fink J et al (2002) Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol 168:3577–3585
Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffmann W (2002) Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem 277:18440–18446
Neveu WA, Allard JL, Raymond DM et al (2010) Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res 11:28
Rubini A (2013) Interleukin-6 and lung inflammation: evidences of a causing role in inducing respiratory system resistance increments. Inflamm Allergy Drug Targets 12(5):315–321
Singhmar P, Huo X, Eijkelkamp N et al (2016) Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci USA 113:3036–3041
Hashimoto A, Tanaka M, Takeda S, Ito H, Nagano K (2015) Cilostazol induces PGI2 production via activation of the downstream Epac-1/Rap1 signaling cascade to increase intracellular calcium by PLCε and to activate p44/42 MAPK in human aortic endothelial cells. PLoS ONE 10:e0132835
Roscioni SS, Kistemaker LE, Menzen MH et al (2009) PKA and Epac cooperate to augment bradykinin-induced interleukin-8 release from human airway smooth muscle cells. Respir Res 10:88
Roscioni SS, Dekkers BG, Prins AG et al (2011) cAMP inhibits modulation of airway smooth muscle phenotype via the exchange protein activated by cAMP (Epac) and protein kinase A. Br J Pharmacol 162:193–209
Liu J, Zhao X, Cao J et al (2011) Differential roles of PKA and Epac on the production of cytokines in the endotoxin-stimulated primary cultured microglia. J Mol Neurosci 45:186–193
Lau WK, Chow AW, Au SC, Ko WH (2011) Differential inhibitory effects of CysLT1 receptor antagonists on P2Y6 receptor-mediated signaling and ion transport in human bronchial epithelia. PLoS ONE 6:e22363
Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM (2007) Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA-and NF-kappaB-independent mechanisms. Cell Signal 19:251–260
Chen C, Du J, Feng W et al (2012) β-Adrenergic receptors stimulate interleukin-6 production through Epac-dependent activation of PKCδ/p38 MAPK signalling in neonatal mouse cardiac fibroblasts. Br J Pharmacol 166:676–688
Ryu KY, Lee HJ, Woo H et al (2019) Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling. J Neuroinflamm 16:190
Lindauer M, Hochhaus A (2014) Dasatinib. Recent Results Cancer Res 201:27–65
Obara Y, Labudda K, Dillon TJ, Stork PJ (2004) PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci 117:6085–6094
Beristain AG, Molyneux SD, Joshi PA et al (2015) PKA signaling drives mammary tumorigenesis through Src. Oncogene 34:1160–1173
Violin JD, Ren XR, Lefkowitz RJ (2006) G-protein-coupled receptor kinase specificity for beta-arrestin recruitment to the beta2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem 281:20577–20588
Yin F, Wang YY, Du JH et al (2006) Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol 40:384–393
Li Y, Dillon TJ, Takahashi M, Earley KT, Stork PJ (2016) Protein kinase A-independent Ras protein activation cooperates with Rap1 protein to mediate activation of the extracellular signal-regulated kinases (ERK) by cAMP. J Biol Chem 291:21584–21595
Singh AK, Vinayak M (2017) Activation of ERK signalling by Src family kinases (SFKs) in DRG neurons contributes to hydrogen peroxide (H2O2)-induced thermal hyperalgesia. Free Radical Res 51:838–850
Acknowledgements
We thank Dr. D.C. Gruenert (Burlington, Vermont, USA) for the generous gift of the 16HBE14o- cells.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.: 82000008), GuangDong Basic and Applied Basic Research Foundation (Grant No.: 2019A1515110126), Medical Scientific Research Foundation of Guangdong Province (Grant No.: A2019335), and Funds for PhD researchers of Guangdong Medical University in 2019 (Grant No.: B2019004) awarded to R.G. Zhang. This work was supported by the Research Grant Council General Research Fund (CUHK 14107920), which was awarded to W.H. Ko.
Author information
Authors and Affiliations
Contributions
R-GZ, YN, K-WP, HP, C-LC, C-YY: Conducted experiments. RGZ: Participated in research design, analyzed and interpreted the data, drafted the manuscript. WK: Interpreted the data, wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declaration of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, RG., Niu, Y., Pan, KW. et al. β2-Adrenoceptor Activation Stimulates IL-6 Production via PKA, ERK1/2, Src, and Beta-Arrestin2 Signaling Pathways in Human Bronchial Epithelia. Lung 199, 619–627 (2021). https://doi.org/10.1007/s00408-021-00484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-021-00484-0