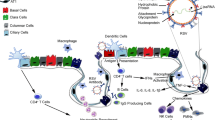
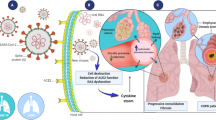
Abstract
The pulmonary epithelium is divided into upper, lower, and alveolar (or small) airway epithelia and acts as the mechanical and immunological barrier between the external environment and the underlying submucosa. Of these, the small airway epithelium is the principal area of gas exchange and has high immunological activity, making it a major area of cell biology, immunology, and pharmaceutical research. As animal models do not faithfully represent the human pulmonary system and ex vivo human lung samples have reliability and availability issues, cell lines, and primary cells are widely used as small airway epithelial models. In vitro, these cells are mostly cultured as monolayers (2-dimensional cultures), either media submerged or at air–liquid interface. However, these 2-dimensional cultures lack a three dimension—a scaffolding extracellular matrix, which establishes the intercellular network in the in vivo airway epithelium. Therefore, 3-dimensional cell culture is currently a major area of development, where cells are cultured in a matrix or are cultured in a manner that they develop ECM-like scaffolds between them, thus mimicking the in vivo phenotype more faithfully. This review focuses on the commonly used small airway epithelial cells, their 2-dimensional and 3-dimensional culture techniques, and their comparative phenotype when cultured under these systems.

Similar content being viewed by others
References
Cardoso WV, Whitsett JA (2008) Resident cellular components of the lung: developmental aspects. Proc Am Thorac Soc 5(7):767–771
Guillot L et al (2013) Alveolar epithelial cells: master regulators of lung homeostasis. Int J Biochem Cell Biol 45(11):2568–2573
Strengert M, Knaus UG (2011) Analysis of epithelial barrier integrity in polarized lung epithelial cells. Methods Mol Biol 763:195–206
Rezaee F, Georas SN (2014) Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol 50(5):857–869
Hollenhorst MI, Richter K, Fronius M (2011) Ion transport by pulmonary epithelia. J Biomed Biotechnol 2011:174306
Samara KD et al (2012) Expression profiles of toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int J Oncol 40(5):1397–1404
Jeyaseelan S et al (2005) Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 32(6):531–539
Bhowmick R et al (2013) Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J Immunol 191(10):5115–5123
Tanaka J et al (2013) Preventive effect of irbesartan on bleomycin-induced lung injury in mice. Respir Investig 51(2):76–83
Mason KM et al (2006) The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol 62(5):1357–1372
Nicholas B et al (2015) A novel lung explant model for the ex vivo study of efficacy and mechanisms of anti-influenza drugs. J Immunol 194(12):6144–6154
Davis AS et al (2015) Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J Histochem Cytochem 63(5):312–328
Gerlach RL et al (2013) Early host responses of seasonal and pandemic influenza A viruses in primary well-differentiated human lung epithelial cells. PLoS ONE 8(11):e78912
Chan RW et al (2010) Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS ONE 5(1):e8713
Stewart CE et al (2012) Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy (Cairo) 2012:943982
Choe MM, Sporn PH, Swartz MA (2003) An in vitro airway wall model of remodeling. Am J Physiol Lung Cell Mol Physiol 285(2):L427–L433
Agarwal AR, Mih J, George SC (2003) Expression of matrix proteins in an in vitro model of airway remodeling in asthma. Allergy Asthma Proc 24(1):35–42
Hall-Stoodley L et al (2006) Mycobacterium tuberculosis binding to human surfactant proteins A and D, fibronectin, and small airway epithelial cells under shear conditions. Infect Immun 74(6):3587–3596
Sisler JD et al (2015) Small airway epithelial cells exposure to printer-emitted engineered nanoparticles induces cellular effects on human microvascular endothelial cells in an alveolar-capillary co-culture model. Nanotoxicology 9:769–779
Escaffre O et al (2013) Henipavirus pathogenesis in human respiratory epithelial cells. J Virol 87(6):3284–3294
Avadhanula V et al (2006) Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 80(4):1629–1636
Kode A, Yang SR, Rahman I (2006) Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 7:132
Dakhama A et al (2003) Induction of regulated upon activation, normal T cells expressed and secreted (RANTES) and transforming growth factor-beta 1 in airway epithelial cells by Mycoplasma pneumoniae. Am J Respir Cell Mol Biol 29(3 Pt 1):344–351
Tiriveedhi V et al (2014) Role of defensins in the pathogenesis of chronic lung allograft rejection. Hum Immunol 75(4):370–377
Homma T et al (2015) Aspergillus fumigatus activates PAR-2 and skews toward a Th2 bias in airway epithelial cells. Am J Respir Cell Mol Biol 54:60–70
Londino JD et al (2015) Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB J 29(7):2712–2725
Hurley BP, Williams NL, McCormick BA (2006) Involvement of phospholipase A2 in Pseudomonas aeruginosa-mediated PMN transepithelial migration. Am J Physiol Lung Cell Mol Physiol 290(4):L703–L709
Hurley BP et al (2004) Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol 173(9):5712–5720
Aufderheide M et al (2013) The CULTEX RFS: a comprehensive technical approach for the in vitro exposure of airway epithelial cells to the particulate matter at the air–liquid interface. Biomed Res Int 2013:734137
Carterson AJ et al (2005) A549 lung epithelial cells grown as three-dimensional aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect Immun 73(2):1129–1140
Gomez-Casal R et al (2013) Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial–mesenchymal transition phenotypes. Mol Cancer 12(1):94
Herzog F et al (2013) Exposure of silver-nanoparticles and silver-ions to lung cells in vitro at the air-liquid interface. Part Fibre Toxicol 10:11
Kaza SK, McClean S, Callaghan M (2011) IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int J Med Microbiol 301(1):26–33
Persoz C et al (2012) Inflammatory response modulation of airway epithelial cells exposed to formaldehyde. Toxicol Lett 211(2):159–163
Barhoumi R et al (2014) Effects of fatty acids on benzo[a]pyrene uptake and metabolism in human lung adenocarcinoma A549 cells. PLoS One 9(3):e90908
Chothia C, Jones EY (1997) The molecular structure of cell adhesion molecules. Annu Rev Biochem 66:823–862
Yan X et al (2013) Identification of CD90 as a marker for lung cancer stem cells in A549 and H446 cell lines. Oncol Rep 30(6):2733–2740
Kreft ME et al (2015) The characterization of the human cell line Calu-3 under different culture conditions and its use as an optimized in vitro model to investigate bronchial epithelial function. Eur J Pharm Sci 69:1–9
Kreda SM et al (2007) Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584(Pt 1):245–259
Fiegel J et al (2003) Large porous particle impingement on lung epithelial cell monolayers—toward improved particle characterization in the lung. Pharm Res 20(5):788–796
Harcourt JL et al (2011) Evaluation of the Calu-3 cell line as a model of in vitro respiratory syncytial virus infection. J Virol Methods 174(1–2):144–149
Garcia-Canton C et al (2013) Metabolic characterization of cell systems used in in vitro toxicology testing: lung cell system BEAS-2B as a working example. Toxicol In Vitro 27(6):1719–1727
Ghio AJ et al (2013) Growth of human bronchial epithelial cells at an air–liquid interface alters the response to particle exposure. Part Fibre Toxicol 10:25
van Schilfgaarde M et al (1995) Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect Immun 63(12):4729–4737
Janmaat ML et al (2006) Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer 118(1):209–214
Vroling AB et al (2007) Allergen induced gene expression of airway epithelial cells shows a possible role for TNF-alpha. Allergy 62(11):1310–1319
Winton HL et al (1998) Cell lines of pulmonary and non-pulmonary origin as tools to study the effects of house dust mite proteinases on the regulation of epithelial permeability. Clin Exp Allergy 28(10):1273–1285
Chen YH et al (2015) Methadone enhances human influenza A virus replication. Addict Biol. doi:10.1111/adb.12305
Newland N, Richter A (2008) Agents associated with lung inflammation induce similar responses in NCI-H292 lung epithelial cells. Toxicol In Vitro 22(7):1782–1788
Chen AI et al (2014) Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog 10(10):e1004480
Chen F et al (2015) Transcriptome profiles of human lung epithelial cells A549 interacting with Aspergillus fumigatus by RNA-Seq. PLoS One 10(8):e0135720
Fulcher ML et al (2009) Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol 296(1):L82–L91
Berman R et al (2014) MUC18 differentially regulates pro-inflammatory and anti-viral responses in human airway epithelial cells. J Clin Cell Immunol 5(5):257–265
Frieke Kuper C et al (2015) Toxicity assessment of aggregated/agglomerated cerium oxide nanoparticles in an in vitro 3D airway model: the influence of mucociliary clearance. Toxicol In Vitro 29(2):389–397
Kastner PE et al (2013) A dynamic system for single and repeated exposure of airway epithelial cells to gaseous pollutants. Toxicol In Vitro 27(2):632–640
Jyonouchi H et al (1998) The effects of hyperoxic injury and antioxidant vitamins on death and proliferation of human small airway epithelial cells. Am J Respir Cell Mol Biol 19(3):426–436
Forbes B, Ehrhardt C (2005) Human respiratory epithelial cell culture for drug delivery applications. Eur J Pharm Biopharm 60(2):193–205
Saatian B et al (2013) Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 1(2):e24333
Coakley RD et al (2003) Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA 100(26):16083–16088
Rusznak C et al (2000) Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 23(4):530–536
Hao Y et al (2012) Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell Microbiol 14(3):401–415
Verriere V et al (2012) Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLoS One 7(5):e37746
Baines KJ et al (2015) Airway beta-defensin-1 protein is elevated in COPD and severe asthma. Mediators Inflamm 2015:407271
Panas A et al (2014) Silica nanoparticles are less toxic to human lung cells when deposited at the air–liquid interface compared to conventional submerged exposure. Beilstein J Nanotechnol 5:1590–1602
Tapparel C et al (2013) Growth and characterization of different human rhinovirus C types in three-dimensional human airway epithelia reconstituted in vitro. Virology 446(1–2):1–8
David J, Sayer NM, Sarkar-Tyson M (2014) The use of a three-dimensional cell culture model to investigate host-pathogen interactions of Francisella tularensis in human lung epithelial cells. Microbes Infect 16(9):735–745
Mathis C et al (2013) Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air–liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol 304(7):L489–L503
Steiner S et al (2012) Cerium dioxide nanoparticles can interfere with the associated cellular mechanistic response to diesel exhaust exposure. Toxicol Lett 214(2):218–225
Thaikoottathil JV et al (2009) Cigarette smoke extract reduces VEGF in primary human airway epithelial cells. Eur Respir J 33(4):835–843
Muckter H et al (1998) A novel apparatus for the exposure of cultured cells to volatile agents. J Pharmacol Toxicol Methods 40(2):63–69
Lenz AG et al (2014) Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air–liquid interface conditions. Am J Respir Cell Mol Biol 51(4):526–535
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8(10):839–845
Liu FF et al (2013) Hanging drop: an in vitro air toxic exposure model using human lung cells in 2D and 3D structures. J Hazard Mater 261:701–710
Hammond TG, Hammond JM (2001) Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol 281(1):F12–F25
Huang S et al (2013) Potential of in vitro reconstituted 3D human airway epithelia (MucilAir) to assess respiratory sensitizers. Toxicol In Vitro 27(3):1151–1156
Wu X et al (2011) Human bronchial epithelial cells differentiate to 3D glandular acini on basement membrane matrix. Am J Respir Cell Mol Biol 44(6):914–921
Wheelock MJ, Johnson KR (2003) Cadherin-mediated cellular signaling. Curr Opin Cell Biol 15(5):509–514
Berger JT et al (1999) Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am J Respir Cell Mol Biol 20(3):500–510
Kleinman HK, Philp D, Hoffman MP (2003) Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol 14(5):526–532
Ding P et al (2014) Transmigration and phagocytosis of macrophages in an airway infection model using four-dimensional techniques. Am J Respir Cell Mol Biol 51(1):1–10
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bhowmick, R., Gappa-Fahlenkamp, H. Cells and Culture Systems Used to Model the Small Airway Epithelium. Lung 194, 419–428 (2016). https://doi.org/10.1007/s00408-016-9875-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9875-2