Abstract
Background
Negative symptoms are the core of schizophrenia, but whether antipsychotics are efficacious for their treatment is unclear. Moreover, there is debate whether patients in relevant trials should have predominant negative symptoms or whether prominent negative symptoms are also acceptable.
Methods
We systematically reviewed randomised, blinded antipsychotic drug trials in patients with schizophrenia and either predominant or prominent negative symptoms (last search Dec 12, 2017). Separate pairwise meta-analyses were conducted in these two populations. The primary outcome was negative symptoms. Depressive, symptoms, positive symptoms, and extrapyramidal side-effects were analysed as causes of secondary negative symptoms.
Findings
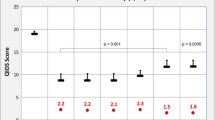
We included 21 randomized-controlled trials with 3451 participants which revealed the following significant differences in the primary outcome: in patients with predominant negative symptoms amisulpride was superior to placebo (N = 4; n = 590, SMD 0.47, CI 0.23, 0.71), olanzapine was superior to haloperidol in a small trial (n = 35) and cariprazine outperformed risperidone (N = 1, n = 456, SMD − 0.29, CI − 0.48, − 0.11). In patients with prominent negative symptoms, olanzapine and quetiapine were superior to risperidone in single trials. Overall, studies in prominent negative symptoms were potentially more confounded by improvements of secondary negative symptoms.
Interpretation
Amisulpride is the only antipsychotic that outperformed placebo in the treatment of predominant negative symptoms, but there was a parallel reduction of depression. Cariprazine was better than risperidone in a large trial that was well-controlled for secondary negative symptoms, but the trial was sponsored by its manufacturer. Future trials should apply scientifically developed definitions such as the deficit syndrome and the persistent negative symptoms concept.



Similar content being viewed by others
References
Buchanan RW, Kirkpatrick B, Heinrichs DW et al (1990) Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 147(3):290–294. https://doi.org/10.1176/ajp.147.3.290
Carpenter WT, Heinrichs JR, Wagman DW AM (1988) Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 145(5):578–583. https://doi.org/10.1176/ajp.145.5.578
Crow TJ (1985) the two-syndrome concept: origins and current status. Schizophr Bull 11(3):471–488. https://doi.org/10.1093/schbul/11.3.471
Strauss JS, Carpenter WT, Bartko JR JJ (1974) The diagnosis and understanding of schizophrenia. Part III. Speculations on the processes that underlie schizophrenic symptoms and signs. Schizophr Bull 11:61–69
Andreasen NC, Olsen S (1982) Negative v positive schizophrenia. Definition and validation. Arch General Psychiatry 39(7):789–794
Marder SR, Galderisi S (2017) The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16(1):14–24. https://doi.org/10.1002/wps.20385
Buchanan RW (2007) Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull 33(4):1013–1022. https://doi.org/10.1093/schbul/sbl057
Leucht S, Leucht C, Huhn M et al. (2017) Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review; bayesian meta-analysis; and meta-regression of efficacy predictors. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.2017.16121358
Leucht S, Arbter D, Engel RR et al (2009) How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14(4):429–447. https://doi.org/10.1038/sj.mp.4002136
Leucht S, Corves C, Arbter D, Engel R, Li C, Davis J (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia. A meta-analysis. Lancet 373:31–41. https://doi.org/10.1016/S0140-6736(08)61764-X
Marder SR, Alphs L, Anghelescu I-G et al (2013) Issues and perspectives in designing clinical trials for negative symptoms in schizophrenia. Schizophr Res 150(2–3):328–333. https://doi.org/10.1016/j.schres.2013.07.058
Leucht S, Huhn M, Rothe P et al (2016) Which are the most important first-generation antipsychotic drugs? Survey of international schizophrenia experts. Abstracts from the 5th Biennial SIRS Conference—Poster Abstracts. NPJ schizophrenia, p 25
Leucht S, Pitschel-Walz G, Engel RR et al (2002) Amisulpride, an unusual “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 159(2):180–190. https://doi.org/10.1176/appi.ajp.159.2.180
Fusar-Poli P, Papanastasiou E, Stahl D et al (2015) Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull 41(4):892–899. https://doi.org/10.1093/schbul/sbu170
Remington G, Foussias G, Fervaha G et al (2016) Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry 3:133–150. https://doi.org/10.1007/s40501-016-0075-8
Elbourne DR, Altman DG, Higgins JPT et al (2002) Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 31(1):140–149
Divine GW, Brown JT, Frazier LM (1992) The unit of analysis error in studies about physicians’ patient care behavior. J General Intern Med 7(6):623–629
Higgins JPTea (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Wiley and Sons, Chichester
Bian ZX, Li YP, Moher D et al. (2006) Improving the quality of randomized controlled trials in Chinese herbal medicine, part I: clinical trial design and methodology. Zhong xi yi jie he xue bao J Chin Integr Med 4(2):120–129
Wu T, Li Y, Bian Z et al (2009) Randomized trials published in some Chinese journals: how many are randomized? Trials 10:46. https://doi.org/10.1186/1745-6215-10-46
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2):261–276
Andreasen NC (1989) The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 155(7):49–58
Kirkpatrick B, Buchanan RW, McKenny PD et al (1989) The schedule for the deficit syndrome. An instrument for research in schizophrenia. Psychiatry Res 30(2):119–123. https://doi.org/10.1016/0165-1781(89)90153-4
Leucht S, Cipriani A, Spineli L et al (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia. A multiple-treatments meta-analysis. Lancet 382(9896):951–962
Furukawa TA, Barbui C, Cipriani A et al (2006) Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 59(1):7–10. https://doi.org/10.1016/j.jclinepi.2005.06.006
Higgins JPT, Jackson D, Barrett JK et al (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3(2):98–110. https://doi.org/10.1002/jrsm.1044
White IR, Barrett JK, Jackson D et al (2012) Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 3(2):111–125. https://doi.org/10.1002/jrsm.1045
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Chaimani A, Salanti G (2015) Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 15(4):905–950
Review Manager (RevMan) (2014) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
Boyer P, Lecrubier Y, Puech AJ et al (1995) Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 166(1):68–72
Buchanan RW, Panagides J, Zhao J et al (2012) Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol 32(1):36–45. https://doi.org/10.1097/JCP.0b013e31823f880a
Danion JM, Rein W, Fleurot O (1999) Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Amisulpride Study Group. Am J Psychiatry 156(4):610–616
Lecrubier Y, Quintin P, Bouhassira M et al (2006) The treatment of negative symptoms and deficit states of chronic schizophrenia. olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta Psychiatr Scand 114(5):319–327
Lindenmayer JP, Khan A, Iskander A et al (2007) A randomized controlled trial of olanzapine versus haloperidol in the treatment of primary negative symptoms and neurocognitive deficits in schizophrenia. J Clin Psychiatry 68(3):368–379
Loo H, Poirier-Littre MF, Theron M et al (1997) Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. Br J Psychiatry 170:18–22
Moller HJ, Riedel M, Muller N et al (2004) Zotepine versus placebo in the treatment of schizophrenic patients with stable primary negative symptoms. a randomized double-blind multicenter trial. Pharmacopsychiatry 37(6):270–278
Németh G, Laszlovszky I, Czobor P et al (2017) Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia. A randomised, double-blind, controlled trial. Lancet 389(10074):1103–1113. https://doi.org/10.1016/S0140-6736(17)30060-0
Alvarez E, Ciudad A, Olivares JM et al (2006) A randomized, 1-year follow-up study of olanzapine and risperidone in the treatment of negative symptoms in outpatients with schizophrenia. J Clin Psychopharmacol 26(3):238–249
Barnas C, Stuppack CH, Miller C et al (1992) Zotepine in the treatment of schizophrenic patients with prevailingly negative symptoms. A double-blind trial vs. haloperidol. Int Clin Psychopharmacol 7(1):23–27
Buchanan RW, Breier A, Kirkpatrick B et al (1998) Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry 155(6):751–760. https://doi.org/10.1176/ajp.155.6.751
Kinon BJ, Noordsy DL, Liu-Seifert H et al (2006) Randomized, double-blind 6-month comparison of olanzapine and quetiapine in patients with schizophrenia or schizoaffective disorder with prominent negative symptoms and poor functioning.[Erratum appears in J Clin Psychopharmacol 26(5) 2009 Apr. 169]. J Clin Psychopharmacol 29(2):453–461
Pichot P, Boyer P (1989) Controlled double blind multi-centre trial of low dose amisulpride versus fluphenazine in the treatment of the negative syndrome of chronic schizophrenia expansion. Scientifique Francaise 125–137
Riedel M, Muller N, Strassnig M et al (2005) Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. Eur Arch Psychiatry Clin Neurosci 255(6):432–437
Ruhrmann S, Kissling W, Lesch OM et al (2007) Efficacy of flupentixol and risperidone in chronic schizophrenia with predominantly negative symptoms. Prog Neuropsychopharmacol Biol Psychiatry 31(5):1012–1022
Saletu B, Kufferle B, Grunberger J et al (1994) Clinical, EEG mapping and psychometric studies in negative schizophrenia. comparative trials with amisulpride and fluphenazine. Neuropsychobiology 29(3):125–135
Sirota P, Pannet I, Koren A et al (2006) Quetiapine versus olanzapine for the treatment of negative symptoms in patients with schizophrenia. Human 21(4):227–234
Speller JC, Barnes TR, Curson DA et al (1997) One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms. Amisulpride v. haloperidol. Br J Psychiatry 171:564–568
Olie JP, Spina E, Murray S et al (2006) Ziprasidone and amisulpride effectively treat negative symptoms of schizophrenia. results of a 12-week, double-blind study. Int Clin Psychopharmacol 21(3):143–151
Soni SD, Mallik A, Schiff AA (1990) Sulpiride in negative schizophrenia. A placebo-controlled double-blind assessment. Hum Psychopharmacol 5(3):233–238
Asada S, Ishimaru T, Kubo S et al (1976) A double-blind study of sulpiride and perphenazine in 82 schizophrenics. Encephale 2(1):73–83
Cesarec Z, Eberhard G, Nordgren L (1974) A controlled study of the antipsychotic and sedative effects of neuroleptic drugs and amphetamine in chronic schizophrenics. A clinical and experimental-psychological study. Acta Psychiatr Scand Suppl 249:65–77
Paillere-Martinot ML, Lecrubier Y, Martinot JL et al (1995) Improvement of some schizophrenic deficit symptoms with low doses of amisulpride. Am J Psychiatry 152(1):130–134
Collins AD, Dundas J (1967) A double-blind trial of amitriptyline/perphenazine, perphenazine and placebo in chronic withdrawn inert schizophrenics. Br J Psychiatry 113(505):1425–1429
Abbas AI, Hedlund PB, Huang X-P et al (2009) Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology 205(1):119–128. https://doi.org/10.1007/s00213-009-1521-8
Boyer P, Lecrubier Y, Stalla-Bourdillon A et al (1999) Amisulpride versus amineptine and placebo for the treatment of dysthymia. Neuropsychobiology 39(1):25–32
Kiss B, Horváth A, Némethy Z et al (2010) Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 333(1):328–340. https://doi.org/10.1124/jpet.109.160432
Jensen NH, Rodriguiz RM, Caron MG et al (2008) N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology 33(10):2303–2312. https://doi.org/10.1038/sj.npp.1301646
Zohar J, Nutt DJ, Kupfer DJ et al (2014) A proposal for an updated neuropsychopharmacological nomenclature. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 24(7):1005–1014. https://doi.org/10.1016/j.euroneuro.2013.08.004
Sagud M, Mihaljevic-Peles A, Begic D et al (2011) Antipsychotics as antidepressants: what is the mechanism? Psychiatr Danub 23(3):302–307
Correll CU, Rubio JM, Inczedy-Farkas G et al (2017) Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74(7):675–684. https://doi.org/10.1001/jamapsychiatry.2017.0624
Helfer B, Samara MT, Huhn M et al (2016) Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry 173(9):876–886. https://doi.org/10.1176/appi.ajp.2016.15081035
Mao Y-M, Zhang M-D (2015) Augmentation with antidepressants in schizophrenia treatment: benefit or risk. Neuropsychiatr Dis Treat 11:701–713. https://doi.org/10.2147/NDT.S62266
Cazorla P, Panagides J, Zhao J et al (2010) Long-term efficacy of asenapine in people with persistent negative symptoms of schizophrenia. Int J Neuropsychopharmcol 13:215. https://doi.org/10.1017/S1461145710000635
Kirkpatrick B, Fenton WS, Carpenter WT et al (2006) The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 32(2):214–219. https://doi.org/10.1093/schbul/sbj053
Levine SZ, Leucht S (2013) Identifying clinically meaningful symptom response cut-off values on the SANS in predominant negative symptoms. Schizophr Res 145(1–3):125–127. https://doi.org/10.1016/j.schres.2012.12.032
Mucci A, Merlotti E, Üçok A et al (2017) Primary and persistent negative symptoms: concepts, assessments and neurobiological bases. Schizophr Res 186:19–28. https://doi.org/10.1016/j.schres.2016.05.014
Acknowledgements
This work was supported by a grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF, Grant Number FKZ 01KG1508). The authors thank Georgia Salanti for the work on the original proposal, Samantha Roberts for help in the literature search and Leonie Reichelt, Hannah Röder, Susanne Bächer and Lio Bäckers for help in data extraction. Special thanks to Rolf Engel, who participated in the development of the project and supervised Marc Krause for his doctoral thesis of which this publication will be part. For full text acquisition and proofreading of the final manuscript, we thank Patricia Kratchowill. We thank all authors of the included studies, particularly those who sent us additional information about their trials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
In the last 3 years, Stefan Leucht has received honoraria for consulting from LB Pharma, Lundbeck, Otsuka, Teva Pharmaceutical Industries Ltd, LTS Lohmann, Geodon Richter, Recordati, Boehringer Ingelheim, and for lectures from Janssen, Lilly, Lundbeck, Otsuka, SanofiAventis and Servier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krause, M., Zhu, Y., Huhn, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 268, 625–639 (2018). https://doi.org/10.1007/s00406-018-0869-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0869-3