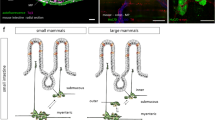
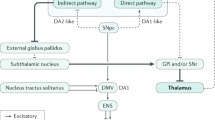
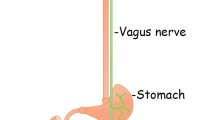
Abstract
Parkinson’s disease (PD) is pathologically characterized by the presence of intraneuronal inclusions, termed Lewy bodies and Lewy neurites, whose main component is alpha-synuclein. Based on the topographic distribution of Lewy bodies and neurites established after autopsy from PD patients, Braak and coworkers hypothesized that PD pathology may start in the gastrointestinal tract then spread through the vagus nerve to the brain. This hypothesis has been reinforced by the discovery that alpha-synuclein may be capable of spreading transcellularly, thereby providing a mechanistic basis for Braak’s hypothesis. This ‘gut to brain’ scenario has ignited heated debates within the movement disorders community and prompted a large number of studies in both humans and animals. Here, we review the arguments for and against the gut as the origin of PD. We conclude that the human autopsy evidence does not support the hypothesis and that it is too early to draw any definitive conclusions. We discuss how this issue might be further addressed in future research.





Similar content being viewed by others
References
Adler CH, Beach TG (2016) Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 31:1114–1119. doi:10.1002/mds.26605
Antelmi E, Donadio V, Incensi A, Plazzi G, Liguori R (2017) Skin nerve phosphorylated α-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology 88:2128–2131. doi:10.1212/WNL.0000000000003989
Antunes L, Frasquilho S, Ostaszewski M, Weber J, Longhino L, Antony P, Baumuratov A, Buttini M, Shannon KM, Balling R, Diederich NJ (2016) Similar α-synuclein staining in the colon mucosa in patients with Parkinson’s disease and controls. Mov Disord 31:1567–1570. doi:10.1002/mds.26702
Attems J, Jellinger KA (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol Appl Neurobiol 34:466–467. doi:10.1111/j.1365-2990.2008.00937.x
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG, Arizona Parkinson’s Disease Consortium (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702. doi:10.1007/s00401-010-0664-3
Beach TG, Corbillé A-G, Letournel F, Kordower JH, Kremer T, Munoz DG, Intorcia A, Hentz J, Adler CH, Sue LI, Walker J, Serrano G, Derkinderen P (2016) multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J Parkinsons Dis 6:761–770. doi:10.3233/JPD-160888
Beach TG, White CL, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH, Arizona Parkinson’s Disease Consortium (2009) Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117:169–174. doi:10.1007/s00401-008-0450-7
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295. doi:10.1111/j.1365-2990.2006.00727.x
Borghammer P (2017) How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord 25:1765. doi:10.1002/mds.27138
Borghammer P, Hamani C (2017) Preventing Parkinson disease by vagotomy: fact or fiction? Neurology 88:1982–1983. doi:10.1212/WNL.0000000000003969
Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach‘s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72. doi:10.1016/j.neulet.2005.11.012
Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 110:517–536. doi:10.1007/s00702-002-0808-2
Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, Jagadapillai R, Liu R, Choe K, Shivakumar B, Son F, Jin S, Kerber R, Adame A, Masliah E, Friedland RP (2016) Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and Caenorhabditis elegans. Sci Rep 6:34477. doi:10.1038/srep34477
Chung SJ, Kim J, Lee HJ, Ryu H-S, Kim K, Lee JH, Jung KW, Kim MJ, Kim YJ, Yun S-C, Lee J-Y, Hong S-M, Myung S-J (2016) Alpha-synuclein in gastric and colonic mucosa in Parkinson’s disease: limited role as a biomarker. Mov Disord 31:241–249. doi:10.1002/mds.26473
Clairembault T, Kamphuis W, Leclair-Visonneau L, Rolli-Derkinderen M, Coron E, Neunlist M, Hol EM, Derkinderen P (2014) Enteric GFAP expression and phosphorylation in Parkinson’s disease. J Neurochem 130:805–815. doi:10.1111/jnc.12742
Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann M-F, Neunlist M, Derkinderen P (2015) Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3:12. doi:10.1186/s40478-015-0196-0
Corbillé A-G, Letournel F, Kordower JH, Lee J, Shanes E, Neunlist M, Munoz DG, Derkinderen P, Beach TG (2016) Evaluation of alpha-synuclein immunohistochemical methods for the detection of Lewy-type synucleinopathy in gastrointestinal biopsies. Acta Neuropathol Commun 4:35. doi:10.1186/s40478-016-0305-8
Davies KN, King D, Billington D, Barrett JA (1996) Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad Med J 72:164–167
Desmet A-S, Cirillo C, Tack J, Vandenberghe W, Vanden Berghe P (2017) Live calcium and mitochondrial imaging in the enteric nervous system of Parkinson patients and controls. Elife 6:197. doi:10.7554/eLife.26850
Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, Naveilhan P, Nguyen J-M, Neunlist M, Derkinderen P (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48. doi:10.1016/j.nbd.2012.09.007
Eberling JL, Dave KD, Frasier MA (2013) α-synuclein imaging: a critical need for Parkinson’s disease research. J Parkinsons Dis 3:565–567. doi:10.3233/JPD-130247
Edwards LL, Quigley EM, Pfeiffer RF (1992) Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 42:726–732
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6:e28032. doi:10.1371/journal.pone.0028032
Fujioka S, Curry SE, Kennelly KD, Tacik P, Heckman MG, Tsuboi Y, Strongosky AJ, van Gerpen JA, Uitti RJ, Ross OA, Ikezu T, Wszolek ZK (2017) Occurrence of Crohn’s disease with Parkinson’s disease. Parkinsonism Relat Disord 37:116–117. doi:10.1016/j.parkreldis.2017.01.013
Fumimura Y, Ikemura M, Saito Y, Sengoku R, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Mizusawa H, Murayama S (2007) Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. J Neuropathol Exp Neurol 66:354–362. doi:10.1097/nen.0b013e3180517454
Hansen C, Angot E, Bergström A-L, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li J-Y, Brundin P (2011) α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121:715–725. doi:10.1172/JCI43366
Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, Hirayama M (2015) Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One 10:e0142164. doi:10.1371/journal.pone.0142164
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32:739–749. doi:10.1002/mds.26942
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C (2013) Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127:235–241. doi:10.1007/s00401-013-1214-6
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang Z-Y, Roybon L, Melki R, Li J-Y (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820. doi:10.1007/s00401-014-1343-6
Hopkins DA, Bieger D, deVente J, Steinbusch WM (1996) Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res 107:79–96
Houser MC, Tansey MG (2017) The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? npj Parkinsons Dis 3:3. doi:10.1038/s41531-016-0002-0
Höglinger GU, Oertel WH, Hirsch EC (2006) The rotenone model of parkinsonism–the five years inspection. J Neural Transm Suppl 70:269–272
Hufnagel DA, Tükel C, Chapman MR (2013) Disease to dirt: the biology of microbial amyloids. PLoS Pathog 9:e1003740. doi:10.1371/journal.ppat.1003740
Hurley D (2015) Does vagotomy reduce the risk for Parkinsonʼs disease? Neurol Today 15:1. doi:10.1097/01.NT.0000475926.46736.74
Ito S, Takao M, Hatsuta H, Kanemaru K, Arai T, Saito Y, Fukayama M, Murayama S (2014) Alpha-synuclein immunohistochemistry of gastrointestinal and biliary surgical specimens for diagnosis of Lewy body disease. Int J Clin Exp Pathol 7:1714–1723
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm (Vienna) 111:1219–1235. doi:10.1007/s00702-004-0138-7
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of alpha-synuclein staging. Neuropathol Appl Neurobiol 34:284–295. doi:10.1111/j.1365-2990.2007.00923.x
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360. doi:10.1002/mds.26307
Klingelhoefer L, Reichmann H (2015) Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat Rev Neurol 11:625–636. doi:10.1038/nrneurol.2015.197
Lebouvier T, Coron E, Chaumette T, Paillusson S, Bruley des Varannes S, Neunlist M, Derkinderen P (2010) Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol Motil 22:e11–e14. doi:10.1111/j.1365-2982.2009.01368.x
Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, Nguyen J-M, Chaumette T, Tasselli M, Paillusson S, Flamand M, Galmiche J-P, Damier P, Derkinderen P (2010) Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One 5:e12728. doi:10.1371/journal.pone.0012728
Lee H-J, Patel S, Lee S-J (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25:6016–6024. doi:10.1523/JNEUROSCI.0692-05.2005
Lee H-J, Suk J-E, Bae E-J, Lee J-H, Paik SR, Lee S-J (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 40:1835–1849. doi:10.1016/j.biocel.2008.01.017
Lee JM, Derkinderen P, Kordower JH, Freeman R, Munoz DG, Kremer T, Zago W, Hutten SJ, Adler CH, Serrano GE, Beach TG (2017) The search for a peripheral biopsy indicator of α-synuclein pathology for Parkinson disease. J Neuropathol Exp Neurol. doi:10.1093/jnen/nlw103
Lin J-C, Lin C-S, Hsu C-W, Lin C-L, Kao C-H (2016) Association between Parkinson’s disease and inflammatory bowel disease: a nationwide taiwanese retrospective cohort study. Inflamm Bowel Dis 22:1049–1055. doi:10.1097/MIB.0000000000000735
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K (2017) Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. doi:10.1212/WNL.0000000000003961
Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM-Y (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA 106:20051–20056. doi:10.1073/pnas.0908005106
Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DMA, Hasegawa M (2013) Prion-like spreading of pathological α-synuclein in brain. Brain 136:1128–1138. doi:10.1093/brain/awt037
Miki Y, Mori F, Wakabayashi K, Kuroda N, Orimo S (2009) Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord 24:2299–2301. doi:10.1002/mds.22775
Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG (2007) Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology 68:2012–2018. doi:10.1212/01.wnl.0000264429.59379.d9
Oyanagi K, Wakabayashi K, Ohama E, Takeda S, Horikawa Y, Morita T, Ikuta F (1990) Lewy bodies in the lower sacral parasympathetic neurons of a patient with Parkinson’s disease. Acta Neuropathol 80:558–559
Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RHW (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5:e8762. doi:10.1371/journal.pone.0008762
Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, Said J, Marsico G, Verbavatz J-M, Rodrigo-Angulo M, Gille G, Funk RHW, Reichmann H (2012) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2:898. doi:10.1038/srep00898
Perez-Pardo P, Dodiya HB, Broersen LM, Douna H, van Wijk N, Lopes da Silva S, Garssen J, Keshavarzian A, Kraneveld AD (2017) Gut-brain and brain-gut axis in Parkinson’s disease models: effects of a uridine and fish oil diet. Nutr Neurosci 123:1–12. doi:10.1080/1028415X.2017.1294555
Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL (2008) Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience 153:733–750. doi:10.1016/j.neuroscience.2008.02.074
Pieri L, Madiona K, Melki R (2016) Structural and functional properties of prefibrillar α-synuclein oligomers. Sci Rep 6:24526. doi:10.1038/srep24526
Postuma RB (2015) Can Parkinson’s disease come from the gut? Mov Disord 30:1325. doi:10.1002/mds.26337
Powley TL, Fox EA, Berthoud HR (1987) Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol 253:R361–R370
Qualman SJ, Haupt HM, Yang P, Hamilton SR (1984) Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 87:848–856
Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut P-O, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E, Vila M (2014) Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75:351–362. doi:10.1002/ana.24066
Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM-Y, Brundin P (2016) Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213:1759–1778. doi:10.1084/jem.20160368
Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K (2017) The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 15:e2000689. doi:10.1371/journal.pbio.2000689
Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S (2004) Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol 63:742–749
Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O (2012) Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci 39:185–188
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(1469–1480):e12. doi:10.1016/j.cell.2016.11.018
Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. doi:10.1002/mds.26069
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH (2012) Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord 27:716–719. doi:10.1002/mds.25020
Shimozawa A, Ono M, Takahara D, Tarutani A, Imura S, Masuda-Suzukake M, Higuchi M, Yanai K, Hisanaga S-I, Hasegawa M (2017) Propagation of pathological α-synuclein in marmoset brain. Acta Neuropathol Commun 5:12. doi:10.1186/s40478-017-0413-0
Sprenger FS, Stefanova N, Gelpi E, Seppi K, Navarro-Otano J, Offner F, Vilas D, Valldeoriola F, Pont-Sunyer C, Aldecoa I, Gaig C, Gines A, Cuatrecasas M, Högl B, Frauscher B, Iranzo A, Wenning GK, Vogel W, Tolosa E, Poewe W (2015) Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology 85:1761–1768. doi:10.1212/WNL.0000000000002126
Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P (2016) Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 79:940–949. doi:10.1002/ana.24648
Stolzenberg E, Berry D, Yang D, Lee EY, Kroemer A, Kaufman S, Wong GCL, Oppenheim JJ, Sen S, Fishbein T, Bax A, Harris B, Barbut D, Zasloff MA (2017) A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun 9:456–463. doi:10.1159/000477990
Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529. doi:10.1002/ana.24448
Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT (2015) Does vagotomy reduce the risk of Parkinson’s disease: the authors reply. Ann Neurol 78:1012–1013. doi:10.1002/ana.24518
Tasselli M, Chaumette T, Paillusson S, Monnet Y, Lafoux A, Huchet-Cadiou C, Aubert P, Hunot S, Derkinderen P, Neunlist M (2013) Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol Motil 25:e183–e193. doi:10.1111/nmo.12070
Tysnes O-B, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH, Reichmann H (2015) Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol 78:1011–1012. doi:10.1002/ana.24531
Ulusoy A, Phillips RJ, Helwig M, Klinkenberg M, Powley TL, Di Monte DA (2017) Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections. Acta Neuropathol 133:381–393. doi:10.1007/s00401-016-1661-y
Ulusoy A, Rusconi R, Pérez-Revuelta BI, Musgrove RE, Helwig M, Winzen-Reichert B, Di Monte DA (2013) Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med 5:1119–1127. doi:10.1002/emmm.201302475
Unger MM, Spiegel J, Dillmann K-U, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer K-H (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. doi:10.1016/j.parkreldis.2016.08.019
Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, Martí C, Serradell M, Lomeña F, Alós L, Gaig C, Santamaria J, Gelpi E (2016) Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol 15:708–718. doi:10.1016/S1474-4422(16)00080-6
Visanji NP, Marras C, Kern DS, Dakheel Al A, Gao A, Liu LWC, Lang AE, Hazrati L-N (2015) Colonic mucosal a-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology 84:609–616. doi:10.1212/WNL.0000000000001240
Visanji NP, Mollenhauer B, Beach TG, Adler CH, Coffey CS, Kopil CM, Dave KD, Foroud T, Chahine L, Jennings D, Systemic Synuclein Sampling Study (S4) (2017) The Systemic Synuclein Sampling Study: toward a biomarker for Parkinson’s disease. Biomark Med 11:359–368. doi:10.2217/bmm-2016-0366
Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM-Y (2011) Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72:57–71. doi:10.1016/j.neuron.2011.08.033
Wagner J, Ryazanov S, Leonov A, Levin J, Shi S, Schmidt F, Prix C, Pan-Montojo F, Bertsch U, Mitteregger-Kretzschmar G, Geissen M, Eiden M, Leidel F, Hirschberger T, Deeg AA, Krauth JJ, Zinth W, Tavan P, Pilger J, Zweckstetter M, Frank T, Bähr M, Weishaupt JH, Uhr M, Urlaub H, Teichmann U, Samwer M, Bötzel K, Groschup M, Kretzschmar H, Griesinger C, Giese A (2013) Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol 125:795–813. doi:10.1007/s00401-013-1114-9
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach‘s and Meissner’s plexuses. Acta Neuropathol 76:217–221
American Association of Neuropathologists, Inc. (2017) Abstracts of the 93rd annual meeting June 8–11, 2017 Garden Grove, CA. J Neuropathol Exp Neurol 76:491–546. doi:10.1093/jnen/nlx029
Acknowledgements
Work in the Derkinderen lab is supported by France Parkinson, Institut de France, CECAP (Comité d’Entente et de Coordination des Associations de Parkinsoniens), FFGP (Fédération française des groupements parkinsoniens) and Parkinsoniens de Vendée. The Arizona group has been supported by the US National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lionnet, A., Leclair-Visonneau, L., Neunlist, M. et al. Does Parkinson’s disease start in the gut?. Acta Neuropathol 135, 1–12 (2018). https://doi.org/10.1007/s00401-017-1777-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1777-8