Abstract
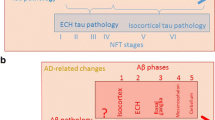
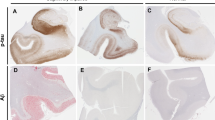
We recommend a new term, “primary age-related tauopathy” (PART), to describe a pathology that is commonly observed in the brains of aged individuals. Many autopsy studies have reported brains with neurofibrillary tangles (NFTs) that are indistinguishable from those of Alzheimer’s disease (AD), in the absence of amyloid (Aβ) plaques. For these “NFT+/Aβ−” brains, for which formal criteria for AD neuropathologic changes are not met, the NFTs are mostly restricted to structures in the medial temporal lobe, basal forebrain, brainstem, and olfactory areas (bulb and cortex). Symptoms in persons with PART usually range from normal to amnestic cognitive changes, with only a minority exhibiting profound impairment. Because cognitive impairment is often mild, existing clinicopathologic designations, such as “tangle-only dementia” and “tangle-predominant senile dementia”, are imprecise and not appropriate for most subjects. PART is almost universally detectable at autopsy among elderly individuals, yet this pathological process cannot be specifically identified pre-mortem at the present time. Improved biomarkers and tau imaging may enable diagnosis of PART in clinical settings in the future. Indeed, recent studies have identified a common biomarker profile consisting of temporal lobe atrophy and tauopathy without evidence of Aβ accumulation. For both researchers and clinicians, a revised nomenclature will raise awareness of this extremely common pathologic change while providing a conceptual foundation for future studies. Prior reports that have elucidated features of the pathologic entity we refer to as PART are discussed, and working neuropathological diagnostic criteria are proposed.



Similar content being viewed by others
References
Ahmed Z, Bigio EH, Budka H et al (2013) Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol 126:537–544. doi:10.1007/s00401-013-1171-0
Alafuzoff I (2013) Alzheimer’s disease-related lesions. J Alzheimers Dis 33(Suppl 1):S173–S179. doi:10.3233/JAD-2012-129024
Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E et al (2013) Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 136:3738–3752. doi:10.1093/brain/awt273
Arai T, Ikeda K, Akiyama H et al (2001) Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 101:167–173
Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1:103–116
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Attems J, Jellinger KA (2006) Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin Neuropathol 25:265–271
Attems J, Lintner F, Jellinger KA (2005) Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis 7:149–157 (discussion 173–180)
Attems J, Thal DR, Jellinger KA (2012) The relationship between subcortical tau pathology and Alzheimer’s disease. Biochem Soc Trans 40:711–715. doi:10.1042/BST20120034
Baborie A, Griffiths TD, Jaros E et al (2012) Frontotemporal dementia in elderly individuals. Arch Neurol 69:1052–1060. doi:10.1001/archneurol.2011.3323
Ball MJ (1978) Topographic distribution of neurofibrillary tangles and granulovacuolar degeneration in hippocampal cortex of aging and demented patients. A quantitative study. Acta Neuropathol 42:73–80
Ball MJ, Nuttall K (1981) Topography of neurofibrillary tangles and granulovacuoles in hippocampi of patients with Down’s syndrome: quantitative comparison with normal ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol 7:13–20
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol 94:403–409
Bancher C, Jellinger KA (1994) Neurofibrillary tangle predominant form of senile dementia of Alzheimer type: a rare subtype in very old subjects. Acta Neuropathol 88:565–570
Bancher C, Paulus W, Paukner K, Jellinger K (1997) Neuropathologic diagnosis of Alzheimer disease: consensus between practicing neuropathologists? Alzheimer Dis Assoc Disord 11:207–219
Beekly DL, Ramos EM, Lee WW et al (2007) The National Alzheimer’s Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord 21:249–258. doi:10.1097/WAD.0b013e318142774e
Berg L, McKeel DW Jr, Miller JP et al (1998) Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 55:326–335
Bondareff W, Mountjoy CQ, Roth M et al (1987) Age and histopathologic heterogeneity in Alzheimer’s disease. Evidence for subtypes. Arch Gen Psychiatry 44:412–417
Bondareff W, Mountjoy CQ, Roth M et al (1987) Neuronal degeneration in locus coeruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord 1:256–262
Bondareff W, Mountjoy CQ, Wischik CM et al (1993) Evidence of subtypes of Alzheimer’s disease and implications for etiology. Arch Gen Psychiatry 50:350–356
Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 4:138–150
Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 153:131–135
Boutajangout A, Wisniewski T (2014) Tau-based therapeutic approaches for Alzheimer’s disease—a mini-review. Gerontology 60(5):381–385. doi:10.1159/000358875
Bowler JV, Hachinski V (1995) Vascular cognitive impairment: a new approach to vascular dementia. Bailliere’s Clin Neurol 4:357–376
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. doi:10.1007/s00401-006-0127-z
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278 (discussion 278–284)
Braak H, Braak E, Bohl J (1993) Staging of Alzheimer-related cortical destruction. Eur Neurol 33:403–408
Braak H, Del Tredici K (2004) Alzheimer’s disease: intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiol Aging 25:713–718. doi:10.1016/j.neurobiolaging.2003.12.015 (discussion 743–716)
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121:171–181. doi:10.1007/s00401-010-0789-4
Braak H, Del Tredici K (2012) Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol 25:708–714. doi:10.1097/WCO.0b013e32835a3432
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. doi:10.1097/NEN.0b013e318232a379
Cairns NJ, Bigio EH, Mackenzie IR et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 114:5–22
Clavaguera F, Akatsu H, Fraser G et al (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. doi:10.1073/pnas.1301175110
Clavaguera F, Bolmont T, Crowther RA et al (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11:909–913. doi:10.1038/ncb1901
Davis DG, Schmitt FA, Wekstein DR, Markesbery WR (1999) Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58:376–388
Dawe RJ, Bennett DA, Schneider JA, Arfanakis K (2011) Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One 6:e26286. doi:10.1371/journal.pone.0026286
de Calignon A, Polydoro M, Suarez-Calvet M et al (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73:685–697. doi:10.1016/j.neuron.2011.11.033
Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45(3):384–389. doi:10.1007/s12031-011-9589-0
Dugger BN, Hidalgo JA, Chiarolanza G et al (2013) The distribution of phosphorylated tau in spinal cords of Alzheimer’s disease and non-demented individuals. J Alzheimers Dis 34:529–536. doi:10.3233/JAD-121864
Dugger BN, Tu M, Murray ME, Dickson DW (2011) Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer’s disease and progressive supranuclear palsy. Neurosci Lett 491(2):122–126. doi:10.1016/j.neulet.2011.01.020
Elobeid A, Soininen H, Alafuzoff I (2012) Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol 123:97–104. doi:10.1007/s00401-011-0906-z
Feany MB, Dickson DW (1996) Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol 40:139–148. doi:10.1002/ana.410400204
Ferrer I, Lopez-Gonzalez I, Carmona M et al (2014) Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol 73:81–97. doi:10.1097/NEN.0000000000000030
Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432. doi:10.1093/brain/awm305
Frost B, Diamond MI (2010) Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11:155–159. doi:10.1038/nrn2786
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284:12845–12852. doi:10.1074/jbc.M808759200
Fukutani Y, Kobayashi K, Nakamura I et al (1995) Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett 200:57–60 (0304-3940(95)12083-G [pii])
Garcia-Sierra F, Hauw JJ, Duyckaerts C et al (2000) The extent of neurofibrillary pathology in perforant pathway neurons is the key determinant of dementia in the very old. Acta Neuropathol 100:29–35
Geser F, Winton MJ, Kwong LK et al (2008) Pathological TDP-43 in parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 115:133–145. doi:10.1007/s00401-007-0257-y
Giannakopoulos P, Hof PR, Mottier S, Michel JP, Bouras C (1994) Neuropathological changes in the cerebral cortex of 1258 cases from a geriatric hospital: retrospective clinicopathological evaluation of a 10-year autopsy population. Acta Neuropathol 87:456–468
Goodman L (1953) Alzheimer’s disease: a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J Nerv Ment Dis 117:97–130
Grinberg LT, Rub U, Ferretti RE et al (2009) The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol 35:406–416. doi:10.1111/j.1365-2990.2009.00997.x
Grinberg LT, Wang X, Wang C et al (2013) Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol 125:581–593. doi:10.1007/s00401-013-1080-2
Grudzien A, Shaw P, Weintraub S et al (2007) Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging 28:327–335. doi:10.1016/j.neurobiolaging.2006.02.007
Guillozet AL, Weintraub S, Mash DC, Mesulam MM (2003) Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 60:729–736
Guo JL, Lee VM (2011) Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286(17):15317–15331. doi:10.1074/jbc.M110.209296
Hauw JJ, Vignolo P, Duyckaerts C et al (1986) Neuropathological study of 12 centenarians: the incidence of Alzheimer type senile dementia is not particularly increased in this group of very old patients. Rev Neurol 142:107–115
Hof PR, Archin N, Osmand AP et al (1993) Posterior cortical atrophy in Alzheimer’s disease: analysis of a new case and re-evaluation of a historical report. Acta Neuropathol 86:215–223
Hof PR, Bouras C, Buée L et al (1992) Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer’s disease cases. Acta Neuropathol 85:23–30
Hof PR, Perl DP, Loerzel AJ, Morrison JH (1991) Neurofibrillary tangle distribution in the cerebral cortex of parkinsonism-dementia cases from Guam: differences with Alzheimer’s disease. Brain Res 564:306–313 (0006-8993(91)91467-F [pii])
Hof PR, Vogt BA, Bouras C, Morrison JH (1997) Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res 37:3609–3625. doi:10.1016/S0042-6989(96)00240-4
Hutton M, Lendon CL, Rizzu P et al (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705. doi:10.1038/31508
Hyman BT, Phelps CH, Beach TG et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. doi:10.1016/j.jalz.2011.10.007
Ikeda K, Akiyama H, Arai T, Nishimura T (1998) Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging 19:S85–S91
Ikeda K, Akiyama H, Arai T et al (1999) Clinical aspects of ‘senile dementia of the tangle type’—a subset of dementia in the senium separable from late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 10:6–11
Ikeda K, Akiyama H, Arai T et al (1997) A subset of senile dementia with high incidence of the apolipoprotein E epsilon2 allele. Ann Neurol 41:693–695. doi:10.1002/ana.410410522
Ikeda K, Akiyama H, Kondo H et al (1995) Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol 90:620–625
Ikeda K, Kondo H, Fujishima T, Kase K, Mizutani Y (1993) A case of atypical senile dementia of Alzheimer type. No To Shinkei 45:455–460
Iseki E, Tsunoda S, Suzuki K et al (2002) Regional quantitative analysis of NFT in brains of non-demented elderly persons: comparisons with findings in brains of late-onset Alzheimer’s disease and limbic NFT dementia. Neuropathology 22:34–39
Ishizawa T, Ko LW, Cookson N et al (2002) Selective neurofibrillary degeneration of the hippocampal CA2 sector is associated with four-repeat tauopathies. J Neuropathol Exp Neurol 61:1040–1047
Itoh Yamada M, Yoshida R et al (1996) Dementia characterized by abundant neurofibrillary tangles and scarce senile plaques: a quantitative pathological study. Eur Neurol 36:94–97
Itoh Y, Yamada M, Suematsu N, Matsushita M, Otomo E (1998) An immunohistochemical study of centenarian brains: a comparison. J Neurol Sci 157:73–81
Jack CR Jr, Albert MS, Knopman DS et al (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:257–262. doi:10.1016/j.jalz.2011.03.004
Jack CR Jr, Knopman DS, Weigand SD et al (2012) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71:765–775. doi:10.1002/ana.22628
Janocko NJ, Brodersen KA, Soto-Ortolaza AI et al (2012) Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol 124:681–692. doi:10.1007/s00401-012-1044-y
Jellinger KA (2013) Challenges in the neuropathological diagnosis of dementias. Int J Neuropathol 1:44
Jellinger KA (2012) Neuropathological subtypes of Alzheimer’s disease. Acta Neuropathol 123:153–154. doi:10.1007/s00401-011-0889-9
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117. doi:10.1007/s00401-006-0156-7
Jellinger KA, Attems J (2010) Prevalence and pathology of vascular dementia in the oldest-old. J Alzheimers Dis 21:1283–1293
Jellinger KA, Bancher C (1998) Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol 8:367–376
Jicha GA, Abner EL, Schmitt FA, et al. (2012) Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging. 33:622 e621–622 e616. doi:10.1016/j.neurobiolaging.2011.02.018
Jicha GA, Parisi JE, Dickson DW et al (2006) Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol 63:674–681
Josephs KA, Whitwell JL, Parisi JE et al (2008) Argyrophilic grains: a distinct disease or an additive pathology? Neurobiol Aging 29:566–573. doi:10.1016/j.neurobiolaging.2006.10.032
Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197:192–193
Knopman DS, Caselli RJ (2012) Appraisal of cognition in preclinical Alzheimer’s disease: a conceptual review. Neurodegener Dis Manag 2:183–195. doi:10.2217/NMT.12.5
Knopman DS, Jack CR Jr, Wiste HJ et al (2013) Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol 73:472–480. doi:10.1002/ana.23816
Knopman DS, Parisi JE, Salviati A et al (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Kovacs GG, Milenkovic I, Wohrer A et al (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126:365–384. doi:10.1007/s00401-013-1157-y
Kovacs GG, Molnar K, Laszlo L et al (2011) A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 122:205–222. doi:10.1007/s00401-011-0819-x
Liu L, Drouet V, Wu JW et al (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7:e31302. doi:10.1371/journal.pone.0031302
Malkani RG, Dickson DW, Simuni T (2012) Hippocampal-sparing Alzheimer’s disease presenting as corticobasal syndrome. Parkinsonism Relat Disord 18:683–685. doi:10.1016/j.parkreldis.2011.11.022
Markesbery WR, Schmitt FA, Kryscio RJ et al (2006) Neuropathologic substrate of mild cognitive impairment. Arch Neurol 63:38–46
Matsui Y, Tanizaki Y, Arima H et al (2009) Incidence and survival of dementia in a general population of Japanese elderly: the Hisayama study. J Neurol Neurosurg Psychiatry 80:366–370. doi:10.1136/jnnp.2008.155481
McKee AC, Cantu RC, Nowinski CJ et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735. doi:10.1097/NEN.0b013e3181a9d503
Mirra SS (1997) The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging 18:S91–S94
Mirra SS, Heyman A, McKeel D et al (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Mitchell TW, Mufson EJ, Schneider JA et al (2002) Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol 51:182–189
Mizutani T, Kasahara M, Yamada S, Mukai M, Amano N (1993) Study on the neuropathological diagnosis of senile dementia of the Alzheimer type. No To Shinkei 45:333–342
Mizutani T, Shimada H (1992) Neuropathological background of twenty-seven centenarian brains. J Neurol Sci 108:168–177
Mizutani T, Shimada H (1991) Quantitative study of neurofibrillary tangles in subdivisions of the hippocampus. CA2 as a special area in normal aging and senile dementia of the Alzheimer type. Acta Pathol Jpn 41:597–603
Montine TJ, Phelps CH, Beach TG et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. doi:10.1007/s00401-011-0910-3
Mungas D, Tractenberg R, Schneider JA, Crane PK, Bennett DA (2014) A 2-process model for neuropathology of Alzheimer’s disease. Neurobiol Aging 35:301–308. doi:10.1016/j.neurobiolaging.2013.08.007
Murray ME, Cannon A, Graff-Radford NR et al (2014) Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. doi:10.1007/s00401-014-1302-2
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796. doi:10.1016/S1474-4422(11)70156-9
Nakaya H, Miki T, Seino S et al (2003) Molecular and functional diversity of ATP-sensitive K+ channels: the pathophysiological roles and potential drug targets. Nihon Yakurigaku Zasshi 122:243–250
Nelson PT, Abner EL, Schmitt FA et al (2009) Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol 68:774–784. doi:10.1097/NEN.0b013e3181aacbe9
Nelson PT, Alafuzoff I, Bigio EH et al (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381. doi:10.1097/NEN.0b013e31825018f7
Nelson PT, Jicha GA, Schmitt FA et al (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66:1136–1146
Noda K, Sasaki K, Fujimi K et al (2006) Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): the Hisayama Study. Neuropathology 26:508–518
Perl DP, Hof PR, Purohit DP, Loerzel AJ, Kakulas BA (2003) Hippocampal and entorhinal cortex neurofibrillary tangle formation in Guamanian Chamorros free of overt neurologic dysfunction. J Neuropathol Exp Neurol 62:381–388
Petersen RC, Aisen P, Boeve BF et al (2013) Criteria for mild cognitive impairment due to alzheimer’s disease in the community. Ann Neurol. doi:10.1002/ana.23931
Petersen RC, Parisi JE, Dickson DW et al (2006) Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 63:665–672. doi:10.1001/archneur.63.5.665
Prestia A, Caroli A, van der Flier WM et al (2013) Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology 80:1048–1056. doi:10.1212/WNL.0b013e3182872830
Ranginwala NA, Hynan LS, Weiner MF, White CL 3rd (2008) Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry 16:384–388. doi:10.1097/JGP.0b013e3181629971
Rijal Upadhaya A, Kosterin I, Kumar S et al (2014) Biochemical stages of amyloid-beta peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer’s disease. Brain 137:887–903. doi:10.1093/brain/awt362
Robinson JL, Geser F, Corrada MM et al (2011) Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain 134:3708–3715. doi:10.1093/brain/awr308
Rohrer JD, Schott JM (2011) Primary progressive aphasia—defining genetic and pathological subtypes. Curr Alzheimer Res 8:266–272 (BSP/CAR/0119 [pii])
Ruben GC, Wang JZ, Iqbal K, Grundke-Iqbal I (2005) Paired helical filaments (PHFs) are a family of single filament structures with a common helical turn period: negatively stained PHF imaged by TEM and measured before and after sonication, deglycosylation, and dephosphorylation. Microsc Res Tech 67:175–195. doi:10.1002/jemt.20197
Sabbagh MN, Sandhu SS, Farlow MR et al (2009) Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer Dis Assoc Disord 23:229–233. doi:10.1097/WAD.0b013e318199d833
Saito Y, Ruberu NN, Sawabe M et al (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63:911–918
Santa-Maria I, Haggiagi A, Liu X et al (2012) The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol 124:693–704. doi:10.1007/s00401-012-1017-1
Schmidt ML, Garruto R, Chen J, Lee VM, Trojanowski JQ (2000) Tau epitopes in spinal cord neurofibrillary lesions in Chamorros of Guam. Neuroreport 11:3427–3430
Schmidt ML, Zhukareva V, Perl DP et al (2001) Spinal cord neurofibrillary pathology in Alzheimer disease and Guam Parkinsonism-dementia complex. J Neuropathol Exp Neurol 60:1075–1086
Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18:691–701. doi:10.3233/JAD-2009-1227
Schnitzler JG (1911) Zur Abgrenzung der sogenannten Alzheimerschen Erkrankung. Z ges Neurol Psychiat. 7:34–57
Schultz C, Ghebremedhin E, Del Tredici K, Rüb U, Braak H (2004) High prevalence of thorn-shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging 25:397–405. doi:10.1016/S0197-4580(03)00113-1
Serrano-Pozo A, Qian J, Monsell SE et al (2014) Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol 75:597–601. doi:10.1002/ana.24125
Serrano-Pozo A, Qian J, Monsell SE et al (2013) Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J Neuropathol Exp Neurol 72:1182–1192. doi:10.1097/NEN.0000000000000016
Shiarli AM, Jennings R, Shi J et al (2006) Comparison of extent of tau pathology in patients with frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17), frontotemporal lobar degeneration with Pick bodies and early onset Alzheimer’s disease. Neuropathol Appl Neurobiol 32:374–387. doi:10.1111/j.1365-2990.2006.00736.x
Simic G, Stanic G, Mladinov M et al (2009) Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol 35:532–554. doi:10.1111/j.1365-2990.2009.01038.x
Sonnen JA, Santa Cruz K, Hemmy LS et al (2011) Ecology of the aging human brain. Arch Neurol 68:1049–1056. doi:10.1001/archneurol.2011.157
Syed A, Chatfield M, Matthews F et al (2005) Depression in the elderly: pathological study of raphe and locus ceruleus. Neuropathol Appl Neurobiol 31:405–413. doi:10.1111/j.1365-2990.2005.00662.x
Takahashi J, Shibata T, Sasaki M, et al (2014) Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer’s disease: high-resolution fast spin-echo T1-weighted imaging. Geriatr Gerontol Int. doi:10.1111/ggi.12280
Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H (2006) The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowl Environ 2006:re1. doi:10.1126/sageke.2006.6.re1
Thal DR, Schultz C, Botez G et al (2005) The impact of argyrophilic grain disease on the development of dementia and its relationship to concurrent Alzheimer’s disease-related pathology. Neuropathol Appl Neurobiol 31:270–279. doi:10.1111/j.1365-2990.2005.00635.x
Thal DR, von Arnim C, Griffin WS et al (2013) Pathology of clinical and preclinical Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 263(Suppl 2):S137–S145. doi:10.1007/s00406-013-0449-5
Togo T, Sahara N, Yen SH et al (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Trachtenberg DI, Trojanowski JQ (2008) Dementia: a word to be forgotten. Arch Neurol 65:593–595. doi:10.1001/archneur.65.5.593
Trojanowski JQ, Ishihara T, Higuchi M et al (2002) Amyotrophic lateral sclerosis/parkinsonism dementia complex: transgenic mice provide insights into mechanisms underlying a common tauopathy in an ethnic minority on Guam. Exp Neurol 176:1–11
Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW (2003) Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol 29:503–510
Ulrich J, Spillantini MG, Goedert M, Dukas L, Staehelin HB (1992) Abundant neurofibrillary tangles without senile plaques in a subset of patients with senile dementia. Neurodegeneration 1:257–284
Vos SJ, Xiong C, Visser PJ et al (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol 12:957–965. doi:10.1016/S1474-4422(13)70194-7
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344:769–772
Wirth M, Villeneuve S, Haase CM et al (2013) Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol 70:1512–1519. doi:10.1001/jamaneurol.2013.4013
Wisniewski HM, Narang HK, Terry RD (1976) Neurofibrillary tangles of paired helical filaments. J Neurol Sci 27:173–181
Yamada M (2003) Senile dementia of the neurofibrillary tangle type (tangle-only dementia): neuropathological criteria and clinical guidelines for diagnosis. Neuropathology 23:311–317
Yamada M, Itoh Y, Sodeyama N et al (1998) Aging of the human limbic system: observations of centenarian brains and analyses of genetic risk factors for senile changes. Neuropathology 18:228–234
Yamada M, Itoh Y, Sodeyama N et al (2001) Senile dementia of the neurofibrillary tangle type: a comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord 12:117–126. doi:10.1159/000051245
Yamada M, Itoh Y, Suematsu N, Otomo E, Matsushita M (1996) Apolipoprotein E genotype in elderly nondemented subjects without senile changes in the brain. Ann Neurol 40:243–245. doi:10.1002/ana.410400217
Yamada M, Itoh Y, Yohinori I et al (1996) Dementia of the Alzheimer type and related dementias in the aged: DAT subgroups and senile dementia of the neurofibrillary tangle type. Neuropathology 16:89–98
Yoshida M (2006) Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology 26:457–470
Yu L, Boyle PA, Leurgans S, Schneider JA, Bennett DA (2014) Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiol Aging 35:819–826. doi:10.1016/j.neurobiolaging.2013.10.074
Acknowledgments
We are extremely grateful to the patients, clinicians, and fellow researchers that made this effort possible. We also acknowledge the following funding sources: the Society for Supporting Research in Experimental Neurology, Vienna, Austria, National Institutes of Health Grants P50AG08702, R01 AG037212, P01AG07232, P30 AG028383, P50 AG005138, P50 AG016574, U01 AG006786, R01 AG041851, R01 AG011378, P30 AG028383, P50 AG016574, P01 AG003949, P30 AG012300, P50 AG005146, P50 AG005136, P50 AG025688, P50 AG005138, P01 AG002219, P50 AG005133, P50 AG005681, P01 AG003991, R01 AG038651, P30 AG019610, P30 AG013854, P30 AG036453, P30 AG010124, AG005131, AG184440, AG008051, Medical Research Council (MRC, G0400074), National Institute for Health Research (NIHR, R:CH/ML/0712), the Dunhill Medical Trust (R173/1110), Alzheimer’s Research UK (ARUK), and the Alzheimer’s Society (AS-PG-2013-011), Louis V. Gerstner, Jr., Foundation, Alzheimer’s Association (NIRG-11-204450), FP7 EU Project Develage (No. 278486), Comprehensive brain research network, Grant-in-Aid for Scientific Research (C; 26430060), and Daiwa Health Science Foundation, BrightFocus Foundation, Alzheimer’s Association NIRGD-12-242642, Alzheimer Forschung Initiative (AFI # 13803) (DRT); German Ministry for Research and Education (BMBF) FTLD-Net, Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Crary, J.F., Trojanowski, J.Q., Schneider, J.A. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128, 755–766 (2014). https://doi.org/10.1007/s00401-014-1349-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1349-0