Abstract
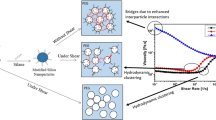
The combined superimposing influences of the surface area of silica nano-particles and molecular weight of polyethylene oxide matrix on the rheological properties of suspensions have been studied. The parameters of both components varied over a wide range: the surface area of silica from 100 to 390 m2/g, molecular weight of poly(ethylene oxide) from 200 to 2 × 105 Da, and concentration of silica from 1 to 13 vol%. In all cases, silica formed aggregates in suspension with apparent diameters of 50 to 230 nm; the higher values were observed for particles with larger surface area. Low-concentration suspensions in an oligomer matrix showed a slight non-Newtonian behavior; the size of silica particles was a determining parameter. Increasing the silica concentration led to dilatancy at high shear stresses. There was a threshold in the concentration dependence of viscosity, beyond which gelation of suspensions occurred. Depending on the silica concentration and molecular weight of the polymeric matrix, the dispersions behaved more like typical colloidal suspensions in a low molecular weight matrix or viscoelastic polymer melts containing an amount of solid filler. An increase in molecular weight of the polymeric matrix resulted in competition between increase in viscosity, appearance of viscoelasticity, and finally the transition from the gel state of a suspension in viscous medium to an elastic fluid.














Similar content being viewed by others
References
Mewis J, Wagner NJ (2012) Colloidal suspension rheology. Cambridge University Press, New York
Malkin AY, Kulichikhin VG (2016) Shear thickening and dynamic glass transition of concentrated suspensions. State of the problem. Colloid J 78:1–8
Ilyin SO, Arinina MP, Malkin AY, Kulichikhin VG (2016) Sol–gel transition and rheological properties of silica nanoparticle dispersions. Colloid J 78:608–615
Malkin A, Kulichikhin V, Ilyin S (2016) A modern look on yield stress fluids. Rheol Acta. doi:10.1007/s00397-016-0963-2
Cohen I, Mason TG, Weitz DA (2004) Shear-induced configurations of confined colloidal suspensions. Phys Rev Lett 93:046001
Guo H, Ramakrishnan S, Hardem JL, Leheny RL (2010) Connecting nanoscale motion and rheology of gel-forming colloidal suspensions. Phys Rev E 81:050401(R)
Seth JR, Mohan L, Locatelli-Champagne C, Cloitre M, Bonnecaze RT (2011) A micromechanical model to predict the flow of soft particle glasses. Nat Mater 10:838–843
Bian X, Litvinov S, Ellero M, Wagner NJ (2014) Hydrodynamic shear thickening of particulate suspension under confinement. J Non-Newton Fluid Mech 213:39–49
Srivastava S, Shin JH, Archer LA (2012) Structure and rheology of nanoparticle-polymer suspensions. Soft Matter 8:4097–4108
Anderson BJ, Zukoski CF (2010) Rheology and microstructure of polymer nanocomposite melts: variation of polymer segment-surface interaction. Langmuir 26:8709–8720
Otsubo Y, Horigome M (2003) Effect of associating polymer on the dispersion stability and rheology of suspensions. Korea-Aust Rheol J 15:27–33
McFarlane NL, Wagner NJ (2010) Poly(ethylene oxide) (PEO) and poly(vinyl pyrolidone) (PVP) induce different changes in the colloid stability of nanoparticles. Langmuir 26:13823–13830
Lafuma F, Wong K, Cabane B (1991) Bridging of colloidal particles through adsorbed polymers. J Colloid Interf Sci 143:9–21
Wong K, Lixon P, Lafuma F, Lindner P, Charriol OA, Cabane B (1992) Intermediate structures in equilibrium flocculation. J Colloid Interf Sci 153:55–72
Russel WB, Saville DA, Schowalter WR (1989) Colloidal dispersions. Cambridge University Press, New York
Ramakrishnan S, Fuchs M, Schweizer KS, Zukoski CF (2002) Entropy driven phase transitions in colloid–polymer suspensions: tests of depletion theories. J Chem Phys 116:2201–2212
Kim S, Hyun K, Moon JY, Clasen C, Ahn KH (2015) Depletion stabilization in nanoparticle–polymer suspensions: multi-length-scale analysis of microstructure. Langmuir 31:1892–1900
Chen YL, Schweizer KS, Fuchs M (2003) Phase separation in suspensions of colloids, polymers and nanoparticles: role of solvent quality, physical mesh, and nonlocal entropic repulsion. J Chem Phys 118:3880–3890
Zhang Z, van Duijneveldt JS (2006) Experimental phase diagram of a model colloid–polymer mixture in the protein limit. Langmuir 22:63–66
Hennequin Y, Evens M, Angulo CMQ, van Duijneveldt JS (2005) Miscibility of small colloidal spheres with large polymers in good solvent. J Chem Phys 123:054906
Zhang Q, Archer LA (2002) Poly(ethylene oxide)/silica nanocomposites: structure and rheology. Langmuir 18:10435–10442
Ha H, Kim SC (2010) Effect of molecular weight of polymer matrix on the dispersion of MWNTs in HDPE/MWNT and PC/MWNT composites. Macromol Res 18:512–518
Kawaguchi M, Mizutani A, Matsushita Y, Kato T (1996) Molecular weight dependence of structures and rheological properties for fumed silica suspensions in polystyrene solutions. Langmuir 12:6179–6183
Choi GN, Krieger IM (1986) Rheological studies on sterically stabilized model dispersions of uniform colloidal spheres: II. Steady-shear viscosity. J Colloid Interf Sci 113:101–113
D’Haene P, Mewis J (1994) Rheological characterization of bimodal colloidal dispersions. Rheol Acta 33:165–174
Rastogi SR, Wagner NJ, Lustig SR (1996) Microstructure and rheology of polydisperse, charged suspensions. J Chem Phys 104:9249–9258
Zhou Z, Solomon M, Scales PJ, Boger DV (1999) The yield stress of concentrated flocculated suspensions of size distributed particles. J Rheol 43:651–671
Li JQ, Salovey R (2004) Model filled polymers: the effect of particle size on the rheology of filled poly(methyl methacrylate) composites. Polym Eng Sci 44:452–462
Le Meins JF, Moldenaers P, Mewis J (2002) Suspensions in polymer melts. 1. Effect of particle size on the shear flow behavior. Ind Eng Chem Res 41:6297–6304
Luckham PF, Ukeje MA (1999) Effect of particle size distribution on the rheology of dispersed systems. J Colloid Interf Sci 220:347–356
Krieger IM, Dougherty TJ (1959) A mechanism for non-Newtonian flow in suspensions of rigid spheres. Trans Soc Rheol 3:137–152
Liu SF, Legrand V, Gourmand M, Lafuma F, Audebert R (1996) General phase and rheological behavior of silica/PEO/water systems. Colloids Surf A Physicochem Eng Asp 111:139–145
Zaman AA (2000) Effect of polyethylene oxide on the viscosity of dispersions of charged silica particles: interplay between rheology, adsorption and surface charge. Colloid Polym Sci 278:1187–1197
Alcantar NA, Aydil ES, Israelachvili JN (2000) Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res 51:343–351
Capuano F, Croce F, Scrosati B (1991) Composite polymer electrolytes. J Electrochem Soc 138:1918–1922
Tao R, Simon SL (2015) Bulk and shear rheology of silica/polystyrene nanocomposite: reinforcement and dynamics. J Polym Sci Pol Phys 53:615–632
Karpukhina EA, Ilyin SO, Makarova VV, Meshkov IB, Kulichikhin VG (2014) Phase state and rheology of polyisobutylene mixtures with decyl surface modified silica nanoparticles. Polym Sci Ser A 56:798–811
Ilyin SO, Polyakova MY, Makarova VV, Meshkov IB, Kulichikhin VG (2016) Phase state and rheology of organosilicon nanocomposites with functionalized hyperbranched nanoparticles. Polym Sci Ser A 58:987–995
Wu F, Zhang S, Chen Z, Zhang B, Yang W, Liu Z, Yang M (2016) Interfacial relaxation mechanisms in polymer nanocomposites through the rheological study on polymer/grafted nanoparticles. Polymer 90:264–275
Yu W, Wang J, You W (2016) Structure and linear viscoelasticity of polymer nanocomposites with agglomerated particles. Polymer 98:190–200
Pauly CS, Genix AC, Alauzun JG, Jestin J, Sztucki M, Mutin PH, Oberdisse J (2016) Structure of alumina-silica nanoparticles grafted with alkylphosphonic acids in poly(ethylacrylate) nanocomposites. Polymer 97:138–146
Dufficy MK, Geiger MT, Bonino CA, Khan SA (2015) Electrospun ultrafine fiber composites containing fumed silica: from solution rheology to materials with tunable wetting. Langmuir 31:12455–12463
Salehiyan R, Song HY, Choi WJ, Hyun K (2015) Characterization of effects of silica nanoparticles on (80/20) PP/PS blends via nonlinear rheological properties from Fourier transform rheology. Macromolecules 48:4669–4679
Huang S, Bai L, Trifkovic M, Cheng X, Macosko CW (2016) Controlling the morphology of immiscible cocontinuous polymer blends via silica nanoparticles jammed at the interface. Macromolecules 49:3911–3918
Parpaite T, Otazaghine B, Caro AS, Taguet A, Sonnier R, Lopez-Cuesta JM (2016) Janus hybrid silica/polymer nanoparticles as effective compatibilizing agents for polystyrene/polyamide-6 melted blends. Polymer 90:34–44
Dil EJ, Favis BD (2015) Localization of micro and nano-silica particles in a high interfacial tension poly(lactic acid)/low density polyethylene system. Polymer 77:156–166
Dil EJ, Favis BD (2015) Localization of micro- and nano-silica particles in heterophase poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer 76:295–306
Spoelstra AB, Goossens JGP (2015) Morphology and rheological properties of silica-filled poly(carbonate)/poly(methyl methacrylate) blends. Polym Eng Sci 55:1951–1959
Zheng Z, Song Y, Yang R, Zheng Q (2015) Direct evidence for percolation of immobilized polymer layer around nanoparticles accounting for sol–gel transition in fumed silica dispersions. Langmuir 31:13478–13487
Wright RAE, Hu B, Henn DM, Zhao B (2015) Reversible sol–gel transitions of aqueous dispersions of silica nanoparticles grafted with diblock copolymer brushes composed of a thermosensitive inner block and a charged outer block. Soft Matter 11:6808–6820
Brunel F, Pochard I, Gauffinet S, Turesson M, Labbez C (2016) Structure and yielding of colloidal silica gels varying the range of interparticle interactions. J Phys Chem B 120:5777–5785
Casalini R, Roland CM (2016) Local and global dynamics in polypropylene glycol/silica composites. Macromolecules 49:3919–3924
Mangal R, Srivastava S, Narayanan S, Archer LA (2016) Size-dependent particle dynamics in entangled polymer nanocomposites. Langmuir 32:596–603
Di Giuseppe E, Davaille A, Mittelstaedt E, François M (2012) Rheological and mechanical properties of silica colloids: from Newtonian liquid to brittle behavior. Rheol Acta 51:451–465
Kostyuk A, Ignatenko V, Smirnova N, Brantseva T, Ilyin S, Antonov S (2015) Rheology and adhesive properties of filled PIB-based pressure-sensitive adhesives. I. Rheology and Shear Resistance. J Adhes Sci Technol 29:1831–1848
Brantseva T, Antonov S, Kostyuk A, Ignatenko V, Smirnova N, Korolev Y, Tereshin A, Ilyin S (2016) Rheological and adhesive properties of PIB-based pressure-sensitive adhesives with montmorillonite-type nanofillers. Eur Polym J 76:228–244
Acknowledgements
This research was partially supported by the Council for Grants of the President of the Russian Federation (project no. MK-545.2017.3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Malkin, A.Y., Ilyin, S.O., Arinina, M.P. et al. The rheological state of suspensions in varying the surface area of nano-silica particles and molecular weight of the poly(ethylene oxide) matrix. Colloid Polym Sci 295, 555–563 (2017). https://doi.org/10.1007/s00396-017-4046-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4046-4