Abstract
Purpose
Inflammation could play a key role in tissue damage and bone metabolism. The modified dietary inflammatory score (M-DIS) is a validated tool to estimate the inflammatory potential of the diet. In the present study, we evaluate the associations between the M-DIS and bone mineral density (BMD) in a senior Mediterranean population with overweight/obesity and metabolic syndrome.
Methods
Baseline cross-sectional association between the M-DIS and bone mineral density was assessed in 1134 participants of the multicenter PREDIMED-Plus trial (aged 55–75 with overweight/obesity and metabolic syndrome). BMD was measured using Dual-energy X-ray Absorptiometry scans and participants answered a food frequency questionnaire to determine the M-DIS. BMD was categorized as low BMD when T score was equal or lower than -1 and normal BMD in another case. Associations between BMD and M-DIS were evaluated by using linear and logistic regressions adjusted by other co-variates.
Results
Participants in the top tertile of the M-DIS had a lower BMD at total femur [β (95% CI) − 0.02 (− 0.04, − 0.01)], trochanter areas [β (95% CI) − 0.03 (− 0.05, − 0.01)] and lumbar spine area [β (95% CI) − 0.03 (− 0.07, 0.01)] (but in the last case, measures were less precise and hence not statistically significant) compared to those in the lower M-DIS tertile. Multiple logistic regression analyses showed that the odds of the total femur and femoral trochanter osteopenia/osteoporosis were higher in participants in the top tertile compared to those in the lowest tertile of M-DIS [OR (95% CI) 1.71 (1.12, 2.64), P for trend 0.015; 2.02 (1.29, 3.21), P for trend 0.002, respectively].
Conclusion
A high pro-inflammatory diet, measured by the M-DIS, is associated with lower BMD in a senior Mediterranean population with metabolic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an age-related chronic disease characterized by the loss of bone mass and trabecular alterations that decline bone strength and increases the risk of fractures. These changes are influenced by non-modifiable factors like genetics and age-related hormone changes, and by modifiable factors related to current lifestyles such as the quality of diet, physical activity, sedentary behaviors, and smoking habits [1, 2].
Aging is the strongest risk factor for the development of musculoskeletal disorders and promotes fat mass accumulation, sarcopenic obesity, bone loss, inflammation, and oxidative stress [3, 4]. Several epidemiological studies have associated systemic inflammation with an increased risk of developing non-communicable chronic diseases, especially obesity, Type 2 Diabetes (T2D), and cardiovascular diseases, but also with osteoporosis, frailty, and sarcopenia [5,6,7,8]. In this regard, it has been suggested that inflammation could play a key role in tissue damage and bone metabolism [9]. Some pro-inflammatory cytokines, such as Tumor Necrosis Factor-alpha (TNF-α) or interleukin 6 (IL-6), may exert an inhibitory effect on osteoprotegerin (OPG) increasing Receptor Activator for Nuclear Factor κ B Ligand (RANKL) concentrations, and consequently, increasing the osteoclastic activity, bone resorption and the risk of osteoporosis incidence [10, 11].
In addition, diet plays a role in the modulation of inflammation and may have a relevant role in the prevention of osteoporosis. Healthy dietary patterns, such as Mediterranean diet (MedDiet), characterized by their high content in fruit, vegetables, whole grains, and fish and that contribute to the intake of nutrients like fiber, omega-3, monounsaturated fatty acids, or vitamin D have been demonstrated to have anti-inflammatory properties and to reduce the bone reabsorption process [12, 13]. Contrary, other studies have linked a higher adherence to pro-inflammatory dietary patterns distinguished by its content in processed food, fats, and red meat with lower bone mineral density (BMD) and increased peripheral inflammation [12,13,14,15].
In this context, the modified dietary inflammatory score (M-DIS) is a validated tool to estimate the inflammatory potential of the diet [16]. Previous studies have shown that higher M-DIS scores are significantly associated with higher circulating inflammatory markers and increased risk of metabolic alterations and chronic diseases such as obesity, metabolic syndrome, T2D, cardiovascular disease, and osteoporosis [16,17,18,19,20]. A recent meta-analysis of epidemiological studies has reported an inverse association between M-DIS and BMD at the lumbar spine and total hip in both men and women, but not at the femoral neck, along with an increased risk of osteoporosis and fractures in those individuals with higher M-DIS scores [21]. Similarly, in a cross-sectional epidemiological study, an inverse correlation between M-DIS and total BMD was reported in a subsample of 121 postmenopausal women [22]. However, the high heterogeneity among the studies included in this meta-analysis, mainly conducted in postmenopausal women with Western diet, the lack of studies conducted in the Mediterranean populations, and other recent publications which did not find associations between these parameters, do necessary to value the usefulness of this score in other contexts where the dietary pattern is considered anti-inflammatory [23, 24]. Therefore, new prospective studies involving different populations, and pooled by sex, are needed to increase the generalization of the result, and the level of evidence of the possible associations between diet, inflammation, and bone status or metabolism.
In the present study, we evaluated the associations between the M-DIS score and bone mineral density in a senior Mediterranean population with overweight/obesity and metabolic syndrome.
Materials and methods
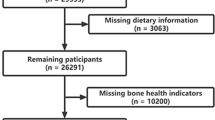
This is a cross-sectional analysis conducted in a subset of 1134 participants from the PREDIMED-Plus study, a multicenter, randomized, and parallel-group clinical trial including 6874 women and men, aged 55–75 with overweight/obesity [body mass index (BMI) between 27 and 40 kg/m2] and metabolic syndrome defined by the updated harmonized criteria of the International Diabetes Federation, the American Heart Association, and the National Heart Association [25]. Participants with severe chronic diseases, drug or alcohol addiction, or allergy to MedDiet food were excluded from the study. For the present study, we have included only randomized individuals with DXA measurements participating in a body composition substudy from 4 of the 23 PREDIMED-Plus recruiting centers: Reus (UNH-URV), Mallorca (Hospital Son Espases/IDISBA), Pamplona (IDISNA) and León (IBIOMED). Participants included in the present analysis with DEXA measurements did not differ from the rest of the participants enrolled in the PREDIMED-Plus trial in terms of age, sex, BMI, and prevalence of obesity and T2D (P > 0.05 for all comparisons).
Detailed protocol and study information was previously published [26] and is available at http://predimedplus.com. Both the protocol and procedures were implemented following the ethical standards of the Declaration of Helsinki and approved by the institutional ethics review boards of each study center (Ref: 13-07-25/7proj2). In addition, all participants provided written informed consent. The PREDIMED-Plus study was registered at http://www.isrctn.com/ (ISRCTN89898870).
Bone assessment
An X-ray Bone Densitometer (DXA) (DXA Lunar Prodigy Primo and Lunar iDXA; GE Healthcare, Madison, WI) was used to assess bone mineral density (BMD) (g/cm2). For this work, we used the following bone areas for their clinical relevance: the total femur (TF), lumbar spine (from L1 to L4) (LS), and trochanter (TR). The BMD at the femoral area was measured on the non-dominant side. The T score of these three measurements (TF, LS, and TR) were calculated using the reference values for the Spanish adult population included in the DXA software considering sex, age, weight, and height of the reference population.
Dietary assessment
Dietary intake was estimated using a validated semi-quantitative food frequency questionnaire (FFQ) composed of 143 items [27]. Energy and nutrient intakes were calculated using Spanish nutritional food composition tables [28, 29]. The method used to estimate the M-DIS scores have been previously described and published [16]. In short, the M-DIS is a score calculated using previously published articles that assessed the effect of 45 food, nutrients (macro and micro), and compounds on several inflammatory biomarkers. Each parameter was scored according to its influence on these inflammatory biomarkers (+ 1 if the parameter increased the inflammatory biomarkers, 0 if it did not have any effect on them, or − 1 if it decreased them). To estimate the individual M-DIS score, the intake of every food item was standardized using the means and SD of each food/nutrient item [16]. The final score is the sum of all its components for each participant. This allows us to classify the participant's diet as an anti-inflammatory (negative values) or pro-inflammatory (positive values) diet. Like previous studies [17], we used the following 32 nutrient/food parameters were used to compute the M-DIS score available in the PREDIMED-Plus study: caffeine (g), alcohol (g), vitamin B1 (mg), vitamin B2 (mg), vitamin B3 (mg), vitamin B6 (mg), vitamin B12 (μg), vitamin A (RE), vitamin C (mg), vitamin E (mg), vitamin D (μg), carbohydrates (g), protein (g), cholesterol (mg), total fatty acids (g), monounsaturated fatty acids (g), polyunsaturated fatty acids (g), saturated fatty acids (g), trans-fatty acids (g), energy intake (kcal), fiber (g), folic acid (μg), garlic (g), iron (mg), magnesium (mg), selenium (μg), zinc (mg), n-3 fatty acids (g), n-6 fatty acids (g), beta-carotene (μg), onions (g), and tea (g).
Other variables measurements
Trained dietitians collected information about lifestyle habits, health status conditions, and medication used. Leisure-time physical activity was evaluated with the validated Spanish version of the Minnesota Leisure-Time Physical Activity Questionnaire [30]. BMI was calculated as weight (kg) divided by the square of height (m2). Both weight and height were measured with calibrated scales and light clothes.
Statistical analysis
Participants were categorized by tertiles of the M-DIS score. Participant characteristics were described according to these tertiles as mean ± standard deviations (SD) when variables were quantitative or percentages (n) when variables were categorical. Differences between tertiles were tested using analysis of variance (ANOVA) or chi-square test, respectively. As total Osteoporosis cases were insufficient to do a statistical analysis considering the typical three categories of BMD state (normal state, Osteopenia state, and Osteoporosis state) without producing a bias, we assessed the association between the M-DIS score and Osteopenia/Osteoporosis status using a dichotomic variable (referred to as “low BMD status”) that was made considering the BMD T scores and a modification of the T score cut-offs established by the World Health Organization (WHO) for each area [2]: low BMD status (1) when the T score of TF, TR, or LS was equal or lower than − 1; normal BMD status (0) when T score values were higher than − 1.
Several models were used to evaluate the association between M-DIS and BMD. Analysis of covariance (ANCOVA) was used to compare differences between tertiles of M-DIS of BMD in TF, TR, and LS. All models were adjusted for sex (woman/man), the prevalence of T2D (yes/no), age (years), BMI (kg/m2), educational level (illiterate/primary education, secondary education, and academic/graduate), smoking status (never/current/former), physical activity (METs/day), use of insulin (yes or no), use of oral antidiabetic drugs (yes or no), use of oral anticoagulants drugs (yes or no), use of calcium and vitamin D supplements (yes or no), and use of estrogens (yes or no), recruitment center and total energy intake (kcal/day). The assumptions of the ANCOVA models were checked using visual or quantitative methods. All graphs and tests yielded models that met the independence of observations, homogeneity of variance, and normality of residuals criteria. Additionally, the Tukey test was used to make multiple comparisons between M-DIS tertiles.
Linear and logistic regression models were fitted to assess the associations between M-DIS and BMD in TF, TR, and LS (linear regression models) or low BMD status (logistic regression). For these analyses, we used tertiles of M-DIS in both linear and logistic models, considering the first tertile (low M-DIS) as the reference. Multivariable models were adjusted for sex (woman/man), the prevalence of T2D (yes/no), age (years), BMI (kg/m2), educational level (illiterate/primary education, secondary education, and academic/graduate), smoking status (never/current/former), physical activity (METs/day), use of insulin (yes or no), use of oral antidiabetic drugs (yes or no), use of oral anticoagulants drugs (yes or no), use of calcium and vitamin D supplements (yes or no), and use of estrogens (yes or no), recruitment center and total energy intake (kcal/day). Like the ANCOVA models, assumptions of the linear and logistic regressions were checked using visual or quantitative methods. All graphs and tests yielded models that met the linearity, independence of errors, homoscedasticity, and normality of residuals criteria.
Stratified logistic regression analyses were conducted in TF, TR, and LS as sensitivity analyses dividing participants by age (< 70 years/ ≥ 70 years), sex, BMI (< 30 kg/m2/ ≥ 30 kg/m2), and T2D. Models were adjusted by the same variables used previously in the main analyses excluding the, respectively, stratified variable.
The P for trend in linear and logistic models was assessed modeling the M-DIS score as a continuous variable. Interactions with sex, T2D, and BMI were evaluated using the likelihood ratio test including the interaction product term as covariables. Because there was no effect significant modifications (P > 0.05), all the analyses were performed with the complete study population.
For these analyses, we used the official PREDIMED-Plus database updated on 17 September 2018. It was considered statistically significant all P values < 0.05. The present statistical analyses were performed with the R software v3.6.1 (www.r-project.org) (R Development Core Team, 2012).
Results
The total sample of participants included in this analysis (48% women) was 65 ± 5 years old, had a mean BMI of 32.6 ± 3.4 kg/m2, reported an average physical activity expenditure of 380 ± 340 MET/day, and an average energy intake of 2469 ± 592 kcal/day. A total of 41% of participants were never smokers, and the prevalence of T2D was 22%. The mean M-DIS of the full sample was -3.87 ± 5.13.
The total number of participants with TF measurements were 1105, for TR 1130, and for LS 985. The distribution of M-DIS was similar between BMD measurements. Compared to participants in the higher BMD tertile, those in the lower tertile showed a mean M-DIS of − 9.61 ± 3.39 for TF, − 9.62 ± 3.50 for TR, and − 9.50 ± 3.55 for LS, while the higher tertiles showed a mean M-DIS of 1.44 ± 2.21 for TF, 1.41 ± 2.39 for TR, and 1.50 ± 2.35 for LS.
Table 1 shows the baseline characteristics of the studied population by tertiles of the M-DIS score. Participants in the lowest M-DIS tertile (highest anti-inflammatory capacity) had higher physical activity and adherence to the MedDiet than those in the top tertile. Education was also significantly different across M-DIS tertiles. No differences across M-DIS tertiles were observed about age, sex, BMI, smoking status, prevalence of T2D, prevalence of osteoporosis, and medication use.
Table 2 shows the beta-coefficients (β) and trends of associations between tertiles of M-DIS score and BMD. Compared to those in the lowest M-DIS tertile, participants in the highest tertile had a lower BMD at total femur [β (95% CI) − 0.02 (− 0.04, − 0.01)], trochanter [β (95% CI) − 0.03 (− 0.05, − 0.01)] and lumbar spine [β (95% CI) − 0.03 (− 0.07, 0.01)] after adjusting for potential confounders. However, the last area was less precise and hence not statistically significant. Moreover, the M-DIS showed a significant inverse association with BMD in the total femur (P value: 0.045), trochanter (P value: 0.012), and lumbar spine (P value: 0.039) areas. Higher M-DIS scores were also associated with lower BMD status in all three BMD measured sites after adjustment for potential confounders [(mean ± SD, T1 vs T3) TF: 1.04 ± 0.15 vs 1.02 ± 0.14, P value: < 0.001; TR: 0.88 ± 0.15 vs 0.85 ± 0.15, P value: < 0.001; LS: 1.19 ± 0.19 vs 1.16 ± 0.21; P value: 0.032].
The distribution of total low BMD status was unlike between BMD measures. The first tertile of TF, TR, and LS had 71 (3 osteoporosis cases and 68 osteopenia cases), 54 (1 osteoporosis case and 53 osteopenia cases), and 99 (13 osteoporosis cases and 86 osteopenia cases) events, respectively. While the third tertile of TF, TR, and LS had 97 (1 osteoporosis case and 96 osteopenia cases), 86 (2 osteoporosis cases and 84 osteopenia cases), and 119 (26 osteoporosis cases and 93 osteopenia cases) events, respectively. The odds ratios (OR) and 95% CI for low BMD status across tertiles of the M-DIS score are shown in Table 3. The M-DIS score was associated with low BMD status in both the TF (OR 1.71, 95% CI 1.12–2.64; P trend: 0.015) and the TR sites (OR 2.02, 95% CI 1.29–3.21; P trend: 0.002) when comparing those participants in the third tertile versus those in the first tertile. Those participants in the top M-DIS tertiles had a non-significant increased odds of low BMD status compared to those in the reference tertile (OR 1.44, 95% CI 0.95–2.17, P trend 0.092).
Results from the sensitivity analyses did not differ from the general ones (Figs. 1, 2 and 3). BMD at the TF showed a significant association between M-DIS's tertiles and low BMD status, when participants were younger than 70 years (OR 1.66, 95% CI 1.02–2.71), presented overweight (OR 2.82, 95% CI 1.17–7.00) and were no diabetics (OR 1.87, 95% CI 1.17–3.03) when comparing those participants in the third versus those in the first tertile. Similarly, BMD at the TR showed significant associations between M-DIS’s tertiles and low BMD status when participants were younger than 70 years (OR 1.90, 95% CI 1.13–3.21), were women (OR 1.97, 95% IC 1.05–3.74), presented overweight (OR 3.41, 95% IC 1.34–9.13), and do not have diabetes (OR 2.18, 95% IC 1.32–3.65). No significant differences in the associations between M-DIS tertiles and low BMD status were found in LS.
Odds ratios (ORs) and 95% confidence intervals (95% CI) for low BMD in total femur comparing tertile 3 versus tertile 1 of the M-DIS score stratified by age, sex, BMI, and T2D status. ORs were adjusted by sex, the prevalence of T2D, age (years), BMI (m/kg2), smoking, education, recruiting center, physical activity (METs/day), use of insulin (yes or no), use of oral antidiabetic drugs (yes or no), use of oral anticoagulants drugs (yes or no), use of vitamin D supplements (yes or no), use of estrogens (yes or no), and energy intake (kcal/day) excluding age, sex, BMI, or T2D status when it is stratified by one of these variables
Odds ratios (ORs) and 95% confidence intervals (95% CI) for low BMD in trochanter comparing tertile 3 versus tertile 1 of the M-DIS score stratified by age, sex, BMI, and T2D status. ORs were adjusted by sex, the prevalence of T2D, age (years), BMI (m/kg2), smoking, education, recruiting center, physical activity (METs/day), use of insulin (yes or no), use of oral antidiabetic drugs (yes or no), use of oral anticoagulants drugs (yes or no), use of vitamin D supplements (yes or no), use of estrogens (yes or no), and energy intake (kcal/day) excluding age, sex, BMI, or T2D status when it is stratified by one of these variables
Odds ratios (ORs) and 95% confidence intervals (95% CI) for low BMD in lumbar spine comparing tertile 3 versus tertile 1 of the M-DIS score stratified by age, sex, BMI, and T2D status. ORs were adjusted by sex, the prevalence of T2D, age (years), BMI (m/kg2), smoking, education, recruiting center, physical activity (METs/day), use of insulin (yes or no), use of oral antidiabetic drugs (yes or no), use of oral anticoagulants drugs (yes or no), use of vitamin D supplements (yes or no), use of estrogens (yes or no), and energy intake (kcal/day) excluding age, sex, BMI, or T2D status when it is stratified by one of these variables
Discussion
In the current cross-sectional analysis, we reported a significant association between a high M-DIS score and a low bone mineral density at different sites (total femur, trochanter, and lumbar spine), along with an increased risk of osteopenia or osteoporosis, in a senior Mediterranean population with metabolic syndrome. Likewise, we observed an inverse association between the risk of osteopenia/osteoporosis and M-DIS in younger participants, without diabetes and with overweight in the femur areas. These results suggest that a pro-inflammatory diet favors an adverse bone environment that promotes bone loss mechanisms.
The M-DIS has been introduced as a tool to link individuals' food intakes with the overall inflammatory potential of their diets [14]. Several epidemiological trials have already investigated the relationship between the M-DIS score and bone health, but up to now, none have assessed the effect of M-DIS in a population with high adherence to the MedDiet (which is already recognized for its anti-inflammatory effect), and presenting with other comorbidities associated with a pro-inflammation state. In our study, the M-DIS score was inversely associated with BMD in both the femur (total femur and trochanter) and lumbar spine (lumbar spine L1–L4) when we used a pooled sample of women and men. A similar trend was observed when we stratified the analysis by sex. These results are consistent with previous studies. In a recent Korean study that included 2778 elderly postmenopausal women aged > 50 years [31], a higher M-DIS score was associated with low femoral BMD. In the Women’s Health Initiative study, lower hip BMD was observed in postmenopausal women with a lower M-DIS score compared to women with a high M-DIS score at baseline, although lower BMD losses were observed after 6 years of follow-up suggesting a possible positive effect of an anti-inflammatory diet [32]. Similarly, in the context of the NHANES (United States National Health and Nutrition Examination Survey Study), the authors reported that an increased M-DIS score was associated with a decreased BMD across most of the measured bone sites (total femur, femoral neck, trochanter, intertrochanter, Wards triangle, total spine, lumbar vertebrae L3, and lumbar vertebrae L4) in both, men and women [33].
Inflammation has been previously associated with bone health and osteoporosis. It is known that inflammatory cytokines can mediate bone loss via stimulation of osteoclast formation and promotion of the OPG/RANK/RANKL pathway [10, 11, 34]. Actually, high serum concentrations of the inflammatory cytokines IL-6 and TNF-α have been associated with osteoporosis [7, 9, 11]. Furthermore, in vitro studies have shown that these molecules are prone to influence osteoclasts by stimulating bone resorption [35,36,37,38].
Diet quality is a modifiable lifestyle factor that can affect bone metabolism [21, 39, 40]. Several studies have assessed the association between the M-DIS score with numerous inflammatory biomarkers [20, 41]. The consumption of some nutrients and dietary patterns considered to be healthy have been associated with better concentrations of cytokines and specific anti-inflammatory bone biomarkers [12, 42, 43], while others, such as carbohydrates and saturated fatty acids, with a pro-inflammatory coefficient in the M-DIS score, have been related to a poorer bone status, osteoporosis development and fractures risk [44]. In our study, people in the first tertile of M-DIS were shown to have higher macronutrients and total energy intake compared to participants in the third tertile. Although these macronutrients are associated with M-DIS pro-inflammatory values, individuals in this category were also observed to have a higher intake of anti-inflammatory micronutrients that could potentially counteract the increased pro-inflammatory compounds. This might contribute to a protective effect on bone metabolism related to a high nutritionally dense diet.
In addition, participants in the highest tertile of M-DIS presented an increased risk of low BMD status (i.e., participants with a higher risk of presenting with osteopenia or osteoporosis) in the femur but not in the lumbar spine. Similarly, in two studies conducted in Korean populations, a high M-DIS score was significantly related to higher ORs for low BMD status (osteopenia cases + osteoporosis cases) in the total femur and femoral neck but not in the lumbar spine [31, 45]. These discrepancies regarding the affected area could be partly explained due to differences in the relationship between BMD with bone mineral content (BMC) and bone area size (BAS) which modify the risk of developing osteoporosis in the spine and hip [46]. It could be speculated that inflammation produces greater effects on larger bones than in smaller bones and, therefore, anti-inflammatory-property diets or diets composed of a large proportion of anti-inflammatory compounds (i.e., diets with negative values of M-DIS) might produce more benefits in terms of bone health for the hip area than the lumbar spine. Additionally, in stratified analyses by T2D, BMI, sex, and age, younger participants with overweight and without diabetes showed an inverse association between M-DIS and the risk of osteopenia/osteoporosis. In this regard, adequately controlled T2D has shown a positive effect on bone health and helped to reverse bone impairments [47, 48], which might partially explain the observed differences between groups. Although we adjusted our models by medication (including diabetic medication), we cannot discard that some of our overweight participants were in a pre-diabetes status without medication that increased the risk of bone damage.
There are other potential mechanisms associated with inflammation that can be boosted by the inflammatory effect of a diet that can be related to bone metabolism. Pro-inflammatory diets might promote glucose and insulin secretion homeostasis disruption, and assist in the chronic activation of pro-inflammatory pathways that benefit the osteoclastogenesis, bone resorption processes, and the disruption of the normal function of osteoblasts [49,50,51]. In the same way, oxidative stress, as an exacerbating circumstance of the inflammatory process, may also have a key role in bone deterioration [52]. High concentrations of reactive oxygen species and reactive nitrogen species derived from the cellular redox process can reduce bone mass and increase apoptosis of osteoblasts by inhibiting osteoblastogenesis and stimulating RANKL and TNF-α secretion [53, 54]. Finally, the antioxidant abilities of processes in the body decline with age, but anti-inflammatory diets with a high quantity of anti-oxidative compounds may help to balance these processes. In accordance with previous investigations, our findings increase the evidence regarding the potential ability of improved diet quality as measured with the M-DIS might help to modulate the inflammatory processes and decrease the risk of bone disease.
Certain limitations of our study are required to be mentioned. First, we cannot establish a cause–effect relation because of the cross-sectional nature of the analyses, and inference between BMD and the M-DIS score is limited. Additionally, we cannot discard a possible role of secondary factors (such as physical activity) despite the adjustment by potential confounders. Second, this study was carried out in an elderly Mediterranean population with metabolic syndrome, and our results cannot be generalized to other populations. Furthermore, we cannot rule out the possibility that low M-DIS (anti-inflammatory diet) values produce enough protective effect on BMD in a population with high adherence to the MedDiet. Additionally, the process of collecting dietary data and computing the M-DIS score without some items of the original score impossible to estimate from FFQs could induce some degree of misclassification (measurement error). This potential measurement error would be likely non-differential because of the prospective design of our study and would address the probable bias of the results towards the null. However, it is known that the use of validated FFQ is a correct methodology in a large perspective [55], especially when trained dietitians assisted the participants to properly complete the FFQ. Finally, the lack of inflammatory markers in our study does not enable us to confirm any potential mechanism that might explain the observed results. However, the major strengths of the study are the use of a DXA scan for measuring BMD, the control for many potential confounding variables, and the inclusion of sensitivity analyses with similar trends.
Conclusions
In conclusion, the results of our study suggest that a high pro-inflammatory diet, measured with an M-DIS score, is associated with lower BMD in a Mediterranean population with metabolic syndrome. Further studies are needed to clarify the potential mechanism that could explain the observed associations.
References
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. https://doi.org/10.1007/s11657-013-0136-1
World Health Organization (2003) Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 921:1–164 (back cover)
Campisi J, Kapahi P, Lithgow GJ et al (2019) From discoveries in ageing research to therapeutics for healthy ageing. Nature 571(7764):183–192. https://doi.org/10.1038/s41586-019-1365-2
Dutra MT, Avelar BP, Souza VC et al (2017) Relationship between sarcopenic obesity-related phenotypes and inflammatory markers in postmenopausal women. Clin Physiol Funct Imaging 37:205–210. https://doi.org/10.1111/CPF.12287
Tamura Y, Omura T, Toyoshima K, Araki A (2020) Nutrition management in older adults with diabetes: a review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients 12:1–29
Tuttle CSL, Thang LAN, Maier AB (2020) Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev 64:101185
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116:1186–1194. https://doi.org/10.1172/JCI28550
Barbour KE, Lui L-Y, Ensrud KE et al (2014) Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res 29:2057–2064. https://doi.org/10.1002/jbmr.2245
Pino AM, Ríos S, Astudillo P et al (2010) Concentration of adipogenic and proinflammatory cytokines in the bone marrow supernatant fluid of osteoporotic women. J Bone Miner Res 25:492–498. https://doi.org/10.1359/jbmr.090802
Gkastaris K, Goulis DG, Potoupnis M et al (2020) Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact 20:372–381
Cauley JA, Danielson ME, Boudreau RM et al (2007) Inflammatory markers and incident fracture risk in older men and women: the health aging and body composition study. J Bone Miner Res 22:1088–1095. https://doi.org/10.1359/jbmr.070409
Movassagh EZ, Vatanparast H (2017) Current evidence on the Association of Dietary Patterns and Bone Health: a scoping review. Adv Nutr 8:1–16. https://doi.org/10.3945/an.116.013326
Casas R, Castro-Barquero S, Estruch R, Sacanella E (2018) Nutrition and cardiovascular health. Int J Mol Sci 19:3988
Stock JK (2019) Cholesterol and inflammatory risk: insights from secondary and primary prevention. Atherosclerosis 280:192–193
Samraj AN, Pearce OMT, Läubli H et al (2015) A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A 112:542–547. https://doi.org/10.1073/pnas.1417508112
Shivappa N, Steck SE, Hurley TG et al (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17:1689–1696. https://doi.org/10.1017/S1368980013002115
Canto-Osorio F, Denova-Gutierrez E, Sánchez-Romero LM et al (2020) Dietary Inflammatory Index and metabolic syndrome in Mexican adult population. Am J Clin Nutr 112:373–380. https://doi.org/10.1093/ajcn/nqaa135
Kim HY, Lee J, Kim J (2018) Association between dietary inflammatory index and metabolic syndrome in the general Korean population. Nutrients. https://doi.org/10.3390/nu10050648
Garcia-Arellano A, Martínez-González MA, Ramallal R et al (2019) Dietary inflammatory index and all-cause mortality in large cohorts: the SUN and PREDIMED studies. Clin Nutr 38:1221–1231. https://doi.org/10.1016/j.clnu.2018.05.003
Shivappa N, Hebert JR, Marcos A et al (2017) Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res 61:1600707
Fang Y, Zhu J, Fan J et al (2020) Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporos Int. https://doi.org/10.1007/s00198-020-05578-8
Arikawa AY, Kurzer MS (2021) Associations between diet quality and anthropometric measures in white postmenopausal women. Nutrients. https://doi.org/10.3390/NU13061947
Herrou J, Julia C, Kesse-Guyot E et al (2020) Absence of association between inflammatory dietary pattern and low trauma fractures: results of the French cohort NutriNet-Santé. Jt Bone Spine 87:632–639. https://doi.org/10.1016/J.JBSPIN.2020.05.013
Cervo MMC, Scott D, Seibel MJ et al (2020) Proinflammatory diet increases circulating inflammatory biomarkers and falls risk in community-dwelling older men. J Nutr 150:373–381. https://doi.org/10.1093/jn/nxz256
Alberti KGMM, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 120:1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644
Martínez-González MA, Buil-Cosiales P, Corella D et al (2019) Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol. https://doi.org/10.1093/ije/dyy225
Fernández-Ballart JD, Piñol JL, Zazpe I et al (2010) Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 103:1808–1816. https://doi.org/10.1017/S0007114509993837
Mataix J (2003) Tablas de composición de alimentos, 4th edn. Universidad de Granada, Granada
Moreiras O, Carvajal A, Cabrera L (2005) Tablas de composición de alimentos food composition tables, 9th edn. Ediciones Pirámide, Madrid
Elosua R, Marrugat J, Molina L et al (1994) Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol 139:1197–1209
Na W, Park S, Shivappa N et al (2019) Association between inflammatory potential of diet and bone-mineral density in Korean postmenopausal women: data from fourth and fifth Korea national health and nutrition examination surveys. Nutrients. https://doi.org/10.3390/nu11040885
Orchard T, Yildiz V, Steck SE et al (2017) Dietary Inflammatory Index, bone mineral density, and risk of fracture in postmenopausal women: results from the women’s health initiative. J Bone Miner Res 32:1136–1146. https://doi.org/10.1002/jbmr.3070
Mazidi M, Shivappa N, Wirth MD et al (2017) The association between dietary inflammatory properties and bone mineral density and risk of fracture in US adults. Eur J Clin Nutr 71:1273–1277. https://doi.org/10.1038/ejcn.2017.133
Wagner D, Fahrleitner-Pammer A (2010) Levels of osteoprotegerin (OPG) and receptor activator for nuclear factor kappa B ligand (RANKL) in serum: are they of any help? Wien Med Wochenschr 160:452–457
Feng W, Liu H, Luo T et al (2017) Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-κB, ERK and JNK signaling pathways. Sci Rep 7:41411. https://doi.org/10.1038/srep41411
Jilka RL, Hangoc G, Girasole G et al (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91
Bertolini DR, Nedwin GE, Bringman TS et al (1986) Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319:516–518. https://doi.org/10.1038/319516a0
Weitzmann MN, Cenci S, Rifas L et al (2001) T cell activation induces human osteoclast formation via receptor activator of nuclear factor κB ligand-dependent and -independent mechanisms. J Bone Miner Res 16:328–337. https://doi.org/10.1359/jbmr.2001.16.2.328
Zeng FF, Xue WQ, Cao WT et al (2014) Diet-quality scores and risk of hip fractures in elderly urban Chinese in Guangdong, China: a case–control study. Osteoporos Int 25:2131–2141. https://doi.org/10.1007/s00198-014-2741-2
Fung TT, McCullough ML, Newby PK et al (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 82:163–173. https://doi.org/10.1093/ajcn.82.1.163
Cavicchia PP, Steck SE, Hurley TG et al (2009) A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 139:2365–2372. https://doi.org/10.3945/jn.109.114025
Martínez-González MA, Salas-Salvadó J, Estruch R et al (2015) Benefits of the mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis 58:50–60. https://doi.org/10.1016/j.pcad.2015.04.003
Hosseini B, Berthon BS, Saedisomeolia A et al (2018) Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr 108:136–155. https://doi.org/10.1093/ajcn/nqy082
Ilich JZ, Kelly OJ, Kim Y, Spicer MT (2014) Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh Hig Rada Toksikol 65:139–148
Kim HS, Sohn C, Kwon M et al (2018) Positive association between dietary inflammatory index and the risk of osteoporosis: results from the KoGES_health examinee (HEXA) cohort study. Nutrients. https://doi.org/10.3390/nu10121999
Deng HW, Xu FH, Davies KM et al (2002) Differences in bone mineral density, bone mineral content, and bone areal size in fracturing and non-fracturing women, and their interrelationships at the spine and hip. J Bone Miner Metab 20:358–366. https://doi.org/10.1007/s007740200052
Athinarayanan SJ, Adams RN, Hallberg SJ et al (2019) Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol (Lausanne). https://doi.org/10.3389/FENDO.2019.00348
Mohsin S, Baniyas MM, Al-Darmaki RS et al (2019) An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin Biol Ther 19:937–948. https://doi.org/10.1080/14712598.2019.1618266
Cunha JS, Ferreira VM, Maquigussa E et al (2014) Effects of high glucose and high insulin concentrations on osteoblast function in vitro. Cell Tissue Res 358:249–256. https://doi.org/10.1007/s00441-014-1913-x
Ghodsi M, Larijani B, Keshtkar AA et al (2016) Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J Diabetes Metab Disord 15:52. https://doi.org/10.1186/s40200-016-0275-1
Martyniak K, Wei F, Ballesteros A et al (2020) Do polyunsaturated fatty acids protect against bone loss in our aging and osteoporotic population? Bone 143:115736
Muller FL, Lustgarten MS, Jang Y et al (2007) Trends in oxidative aging theories. Free Radic Biol Med 43:477–503
Liu H, Bian W, Liu S, Huang K (2012) Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol Trace Elem Res 150:441–450. https://doi.org/10.1007/s12011-012-9488-4
Chen JR, Lazarenko OP, Shankar K et al (2010) A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/β-catenin signaling. J Bone Miner Res 25:1117–1127. https://doi.org/10.1002/jbmr.7
Satija A, Yu E, Willett WC, Hu FB (2015) Understanding nutritional epidemiology and its role in policy. Adv Nutr 6:5–18. https://doi.org/10.3945/AN.114.007492
Acknowledgements
We acknowledge and appreciate all the work of the PREDIMED-Plus personnel, and are grateful for all support received from Rovira i Virgili University (URV), Pere Virgili Institute (IISPV), and Sant Joan Hospital.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The PREDIMED-Plus study was supported by the Fondo de Investigación para la Salud (FIS), which is co-funded by the European Regional Development Fund of the Spanish Ministry of Health (ISCIII) (PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158); the European Research Council (Advanced Research Grant 2013–2018, 340918); the Recercaixa grant 2013 (2013ACUP00194); the Consejería de Salud de la Junta de Andalucía (PI0458/2013); and the Semergen grant; the National Institutes of Health (R01DK127601); the ‘FOLIUM’ programme within the FUTURMed project from the Fundación Instituto de Investigación Sanitaria Illes Balears (financed by 2017annual plan of the sustainable tourism tax and at 50% with charge to the ESF Operational Program 2014–2020 of the Balearic Islands) to J. Konieczna. Consorcio Ciber de Obesidad y Nutrición (CIBEROBN), Consorcio Ciber de Epidemiología y Salud Pública (CIBERESP), and Consorcio de Diabetes y Enfermedades Metabólicas asociadas (CIBERDEM) are initiatives of ISCIII, Spain. Jordi Salas-Salvadó, senior author of this study, is partially supported by ICREA under the ICREA Academia programme. JFG-G has received the Contratos Pre-doctorales de Formación en Investigación en Salud (PFIS FI17/00255) of the Acción Estratégica en Salud program (AES) from the Carlos III Health Institute (ISCIII), Spanish Ministry of Health. IP-G receives a grant from the Ministerio de Ciencia, Innovación y Universidades (MICINN) (FPU 17/01925).
Author information
Authors and Affiliations
Contributions
JS-S, DR, JAM, VM, MAM-G designed research. JFG-G, IP-G, JK, JAdPF conducted research. JS-S, DR, JAM, VM, MAM-G were the coordinators of subject recruitment at the outpatient clinics. JFGG, IP-G analyzed the data. JFGG, IP-G, NB, JS-S, MB interpreted statistical analysis and data. JFGG, IP-G, MB, MR-C, AG, MAM drafted the paper. JS-S, DR, JAM, VM, MAM-G supervised the study and JS-S, DR, JAM, VM, MAM-G had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
JS-S reports serving on the board of and receiving grant support through his institution from the International Nut and Dried Fruit Council and Eroski Foundation. Reports serving in the Executive Committee of the Instituto Danone Spain and on the Scientific Committee of the Danone International Institute. He has received research support from Patrimonio Comunal Olivarero, Spain, and Borges S.A., Spain. Reports receiving consulting fees or travel expenses from Danone, Eroski Foundation, Instituto Danone—Spain, and Abbot Laboratories.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Gavilán, J.F., Paz-Graniel, I., Babio, N. et al. Inflammatory potential of diet and bone mineral density in a senior Mediterranean population: a cross-sectional analysis of PREDIMED-Plus study. Eur J Nutr 61, 1445–1455 (2022). https://doi.org/10.1007/s00394-021-02751-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02751-5